Bats: General Characteristics and Classification
Bats are small mammals capable of true sustained flight.
They belong to the second-largest mammalian order, Chiroptera (order
of mammals adapted for flight), which comprises 22% (over 1400) of all
mammalian species after rodents. They are found on all continents except
Antarctica. Bats are divided into two types: Megachiroptera (fruit
bats that rely mainly on vision), which fly by vision, and Microchiroptera
(insectivorous bats), which fly by echolocation (biological
sonar using sound waves) and magnetoreception (ability to sense
Earth’s magnetic field).
Habitat, Ecology, and Ecological Importance of Bats
Most bats live in caves, hollow trees, and foliage, in large
colonies of 100 to 100,000 individuals. Because of their feeding practices on
insects, fruits, nectar, pollen, fish, etc., they are extremely important for
the ecosystem, for pollination and pest control. Without bats, numerous
medicinal plants would vanish.
Unique Biological Features of Bats
Bats are rather unique mammals. Their ability to harbor
viruses comes from various traits within themselves. A few of those traits are
as follows:
• Bats can fly up to 2000 km, depending on the species, in
their immense migration movements.
• During their flight, bats are metabolically active with
their body temperature rising to about 41°C, with 1066 beats per minute and up
to 34 times basal metabolic rate owing to energy consumption.
• Because of this reason, the nocturnal mammals sleep and
hibernate occasionally, during which their heart beats become 10 to 16 beats
per minute, with the body temperature being less than 5.8°C.
• Similarly, despite their smaller body size ratio, they are
the second most common living mammal, living up to 40 years.
Bats as Viral Host Reservoirs
The idea of viruses being in bats was formulated in the
early 20th century with the discovery of the rabies virus (neurotropic
RNA virus) in bats. Since then, about 54% of zoonotic viral pathogens
(viruses transmitted from animals to humans) have been discovered in
these small creatures. A few of the significant bat-borne viruses are SARS,
MERS, Ebola, Hendra, Nipah, etc. These viruses are responsible for respiratory
diseases, diarrhea, pneumonia, bronchitis, the common cold, and other diseases.
Coronaviruses (enveloped RNA viruses) like SARS-CoV-2
(virus causing COVID-19) are asymptomatic in bats, unlike in civets and
pangolins. However, they are not entirely immune to all illnesses. To
elaborate, a few viruses, such as Tacaribe virus (arenavirus) and
Lyssavirus (rabies-related virus), can cause severe symptoms in
bats, even leading to death.
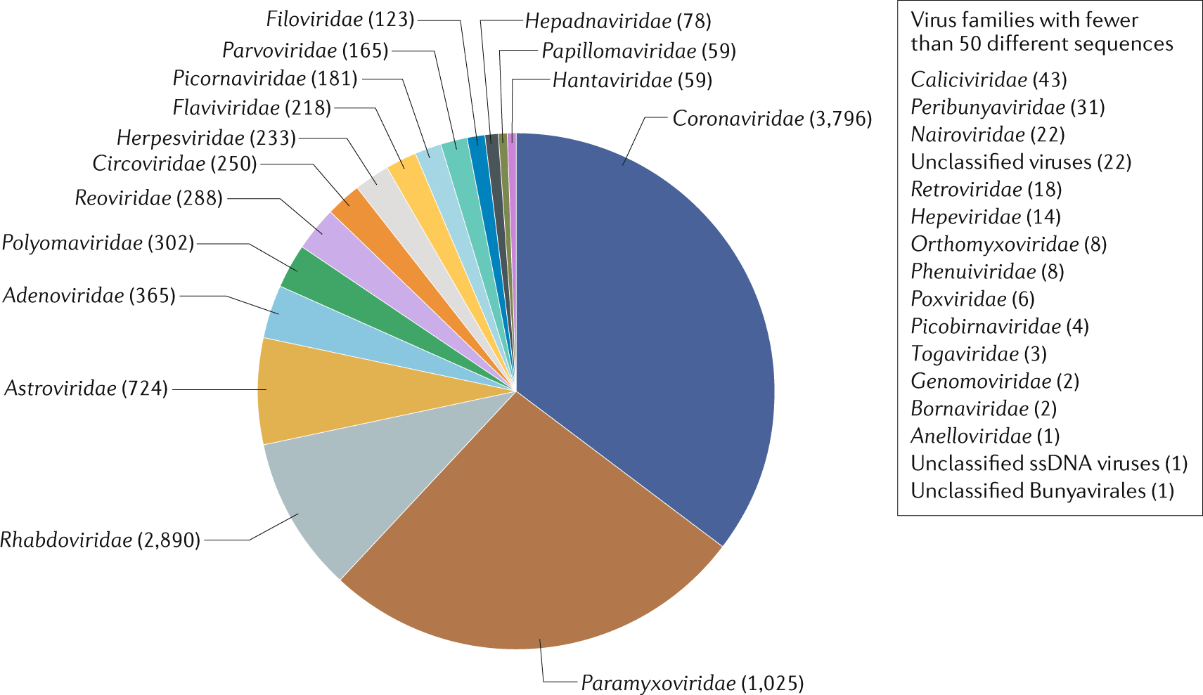
Figure: Diversity of Zoonotic viruses in bats. Source:
Irving et al., 2021.
Coevolution of Bats and Viruses
The abundance of viruses in bats calls for their
relationship to be rooted deep within their genome, relating to evolution.
Fossil evidence suggests that bats and viruses go way back to more than 65
million years during the extinction of dinosaurs, i.e., the KT extinction
event (Cretaceous–Tertiary mass extinction). Studies have revealed
that bats have an abundance of RNA viruses (viruses with RNA genomes)
rather than DNA viruses (viruses with DNA genomes).
This might be because RNA viruses are more prone to errors
because of the lack of a proofreading mechanism (error correction
during replication) in RNA polymerase (enzyme that synthesizes
RNA), in contrast to DNA polymerase (enzyme that synthesizes DNA)
in DNA viruses. This leads to more variability in the antigen receptor (viral
structure recognized by the immune system) of the virus, which enhances its
viral capabilities to infect the host.
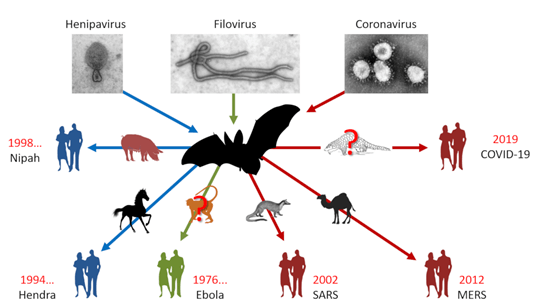
Figure: Zoonotic Transmission of Virus from Bats. Source:
Gérald, 2020.
Bat Immunity Against Viruses
Several hypotheses exist regarding why bats harbor a
considerable number of viruses. Since the discovery of viral pathogens in bats,
the fever hypothesis (idea that elevated body temperature limits
viral replication) has been thought to be the major assumption for bat
immunity.
When the body gets infected by a certain pathogen, its
primary defense mechanism is to raise temperature, to cause fever. The body
increases its temperature to a suboptimal level to kill the unwanted
pathogen/s. Bats innately have rising body temperatures of up to 41°C during
their flight. According to the fever hypothesis, this is thought to decrease
viral load and minimize infection. However, research has suggested that no
viral titer variability was found in bat cells grown at 37°C and 41°C, meaning that
the number of virus counts was similar in both cases. The hypothesis, however,
cannot be fully discarded as it might have some kind of relationship with other
aspects of bat immunity.
Innate Defense Mechanisms in Bats
• Interferon Expression: Type I Interferons (IFNs)
(antiviral cytokines) are cytokines or signaling proteins that help the
body fight against infections. In humans, IFNs are inducible after infection.
The same is not true for bats; bats constitutively express IFNs even before
stimulation with enhanced viral response. These genes are regulated by IFN
regulatory factors (transcription factors controlling IFN genes),
lowering the production of inflammatory cytokines. Similarly, IFN-stimulated
genes (ISGs) (genes activated by interferons) also play a role in
maintaining viral titer in bats without showing any sign of clinical symptoms.
• Heat shock proteins (HSPs) (molecular chaperones
that protect proteins) are produced in enhanced levels in bats and tolerate
high temperature and oxidative stress during flight. This may account for the
rapid evolution of the virus, which tolerates mutations through the use of
chaperone viral proteins.
• Enhanced autophagy (cellular recycling and
pathogen removal mechanism) is well developed in bats and helps in removing
pathogens.
Immune Tolerance Mechanisms in Bats
The most significant immune tolerance mechanisms in bats are
the variation in the cGAS–STING pathway (DNA sensing innate immune
pathway) and the NLRP3 inflammasome pathway (inflammatory
cytokine activation pathway).
cGAS–STING Pathway
The cGAS–STING pathway is a component of the immune
system that functions to determine the presence of cytosolic DNA to trigger the
expression of inflammatory genes. Foreign or abnormal DNA is initially detected
by cGAS (cyclic GMP-AMP synthase) (DNA sensor enzyme). The cGAS
binds to the DNA and leads to the formation of cGAMP (second
messenger molecule). The produced cGAMP binds to STING (Stimulator of
Interferon Genes) (adapter protein) and activates it. This
activation leads to signaling events involving TBK1 (kinase) and IRF3
(transcription factor), resulting in IFN production.
In bats, however, the STING-dependent IFN response is weak
due to a point mutation in the STING protein, inducing weak TBK1 response and
leading to lower production of IFN.
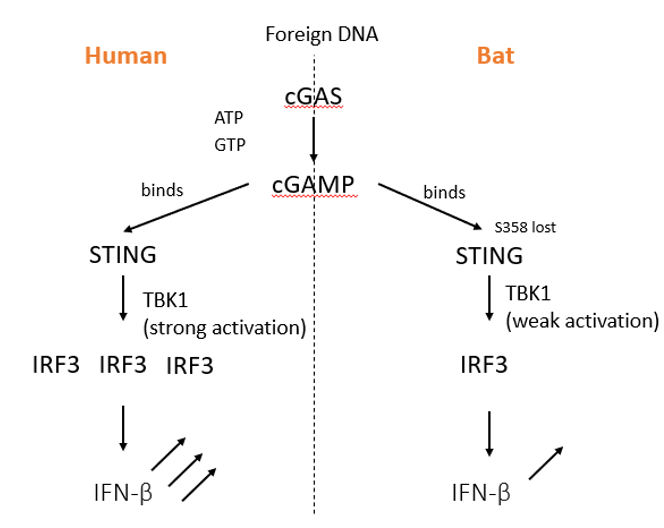
Figure: Comparison between the cGAS–STING Pathway in
Humans and Bats.
NLRP3 Inflammasome Pathway
The NLRP3 inflammasome pathway regulates the innate
immune system and inflammatory cytokines.
In humans or mice, the pathway involves activation of NF-κB
signaling (transcription factor pathway) via Pattern Recognition
Receptors (PRRs) (pathogen sensors), leading to expression of pro-IL-1β
and NLRP3. The ASC protein (adaptor protein) recruits caspase-1
(protease enzyme), which cleaves pro-IL-1β to IL-1β, inducing
inflammation and pyroptosis (inflammatory cell death).
In bats, the inflammasome pathway is diminished. PRR
activation abnormally activates NLRP3, leading to reduced protein function.
Loss of AIM2 protein (DNA sensor of PYHIN family) impairs ASC
complex formation, resulting in reduced caspase-1 activation. Therefore,
pyroptosis is absent and IL-1β production is lowered, significantly reducing
inflammation.
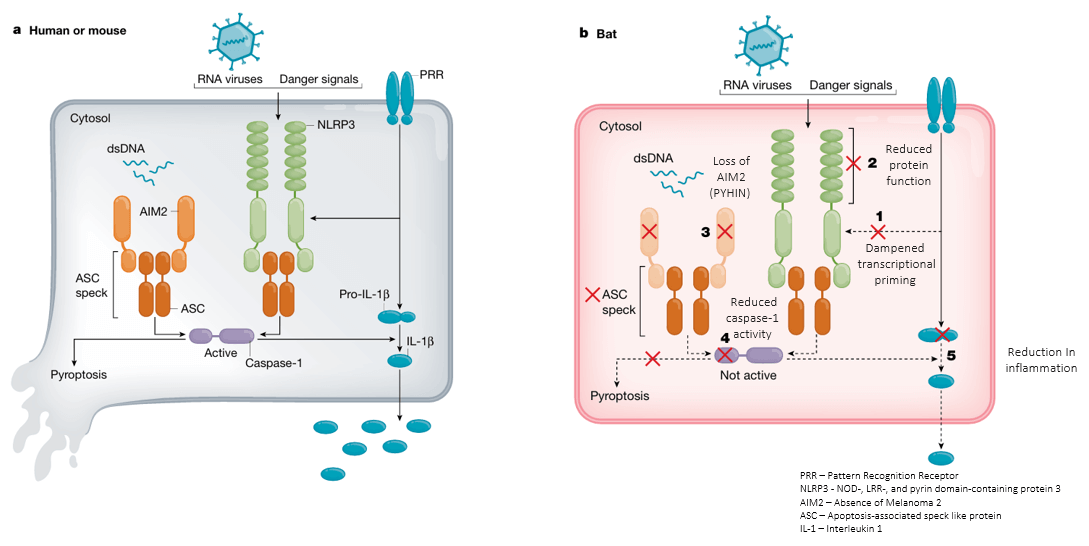
Figure: Comparison between the NLRP3 Inflammasome pathway in Human/mouse and Bat. Source: Irving et al., 2021.
Spillover in Bats
• Roosting habitat: Bats innately have a dirty
roosting habitat where thousands of bats reside in a single cave. They often
excrete, which becomes a grooming area for zoonotic pathogens (pathogens
transmitted from animals to humans). The pathogens can also spread easily
within the colony.
• Physiological stress: When bats are stressed by
certain stimuli, which can include low food availability or a predator, their
behavior becomes abnormal. This leads to reduced immune function, more
susceptibility to pathogens, and negatively affects reproductive physiology
(biological processes related to reproduction).
• Environmental changes: Climate change (long-term
alteration of temperature and weather patterns) affects the physiology of
the ecosystem. Bats must travel large distances to feed on insects, fruits,
nectar, etc., which is not optimal. Similarly, urbanization (expansion
of human settlements) disturbs bat habitats, therefore promoting viral
spillover.
• Direct human exposure: Direct human exposure to
bats is rare. In some parts of the world, humans hunt bats for illegal wildlife
trading and even consume them. Bat excreta, known as bat guano (nitrogen-rich
bat feces), is also a valuable fertilizer because of its rich nitrogen
content. The extraction of this compound can expose humans to bats, leading to zoonotic
transmission (animal-to-human disease spread).
• Bridging host exposure: Bridging host exposure is
more common when an intermediate host (animal transmitting pathogens
between species) directly contacts bats, and then the pathogen is
transmitted to humans. This is exemplified by the SARS-CoV-2 pandemic,
where civets or pangolins were proposed to be infected through bats and
subsequently transmitted the virus to humans.
Learning from Bats
• Immune tolerance: Bats have developed several
mechanisms to combat zoonotic viral pathogens and inflammatory diseases.
Studies on these mechanisms can be helpful in developing cures for autoimmune
diseases (conditions where the immune system attacks self tissues)
or combating viral infections.
• Viral spillovers: Research on viral spillovers from
bats or other zoonoses (diseases transmitted from animals to humans)
helps prevent and predict potential epidemics and pandemics in the future.
• Model species: Like mouse, zebrafish (vertebrate
genetic model), Drosophila (fruit fly genetic model), bats
have high potential to be powerful model species (organisms used to
study biological processes) to study zoonotic hosts. Bat-mouse chimera
models (organisms containing cells from two species) and mouse
models with bat genes are being developed to study zoonotic host–pathogen
dynamics.
• Disturbance of bat habitats: Because of bat habitat
characteristics and their association with multiple diseases, it is wise to
avoid contact with bats or disturbance of their populations.
Challenges in Bat Research
• In vivo challenges: In vivo research (experiments
conducted in living organisms) is essential to fully understand bats and
their physiology. This requires captivity of bats, which is extremely
challenging due to their nocturnal behavior, flight ability, and mysterious
lifestyle. Extensive safety measures and care are required.
• In vitro challenges: In vitro research (experiments
conducted outside living organisms) is an alternative approach. Culturing
bat cells in laboratory conditions is developing but remains in a novice stage.
This requires bat-specific tools, reagents, and antibodies. Due to differences
in bat immunity compared to humans and mice, appropriate immunological
reagents (tools used to study immune responses) are limited.
• Larger species diversity: Because of extensive species diversity (variety of species within a group) in bats, identifying a consensus bat species for research is difficult. Findings in one bat species may not apply to others.






![LATEX TEST FOR RHEUMATOID ARTHRITIS [RHEUMATOID FACTOR]](https://examtube.in/public/assets/images/blog/LNKhD-rheumatoid-factor.jpg)