Introduction
Determining DNA quantity and purity is important prior to other reactions (PCR) for which it is necessary to know the exact DNA concentration in the sample of isolated DNA. DNA is estimated by various methods like UV spectrophotometry. The amount of UV radiation absorbed by a solution of DNA is directly proportional to the amount of DNA in the sample usually absorbance is measured at 260nm, an absorbance of 1.0 corresponds to 50 microgram of double-stranded DNA per ml. another method include diphenylamine reaction. The principle of DPA method is as follow.
Principle
This is a general reaction given by deoxypentoses. The 2-deoxyribose of DNA, in the presence of acid, is converted to ω-hydroxylevulinic aldehyde which reacts with diphenylamine to form a blue colored complex with absorbance maxima at 595 nm. Compounds such as furfural alcohol and arabinal, which can be converted into ω -hydroxylevulinic aldehyde will also give this reaction. In DNA, since only deoxyribose of purine nucleotides is released, the value obtained represents half of the total deoxyribose in the sample. The reactions leading to the formation of the coloured complex are as follows

Requirements
- Standard DNA solution (250 microgram/ml)
- Unknown DNA sample solution
- Diphenylamine (DPA) reagent
- Distilled water
- Spectrophotometer
- Boiling water bath
- Pipettes, Test-tube, Graph paper
Procedure
Arrange the tubes as shown in table to prepare standard curve and add the reagents according to the table. Similarly prepare tubes for sample and precede as per table.
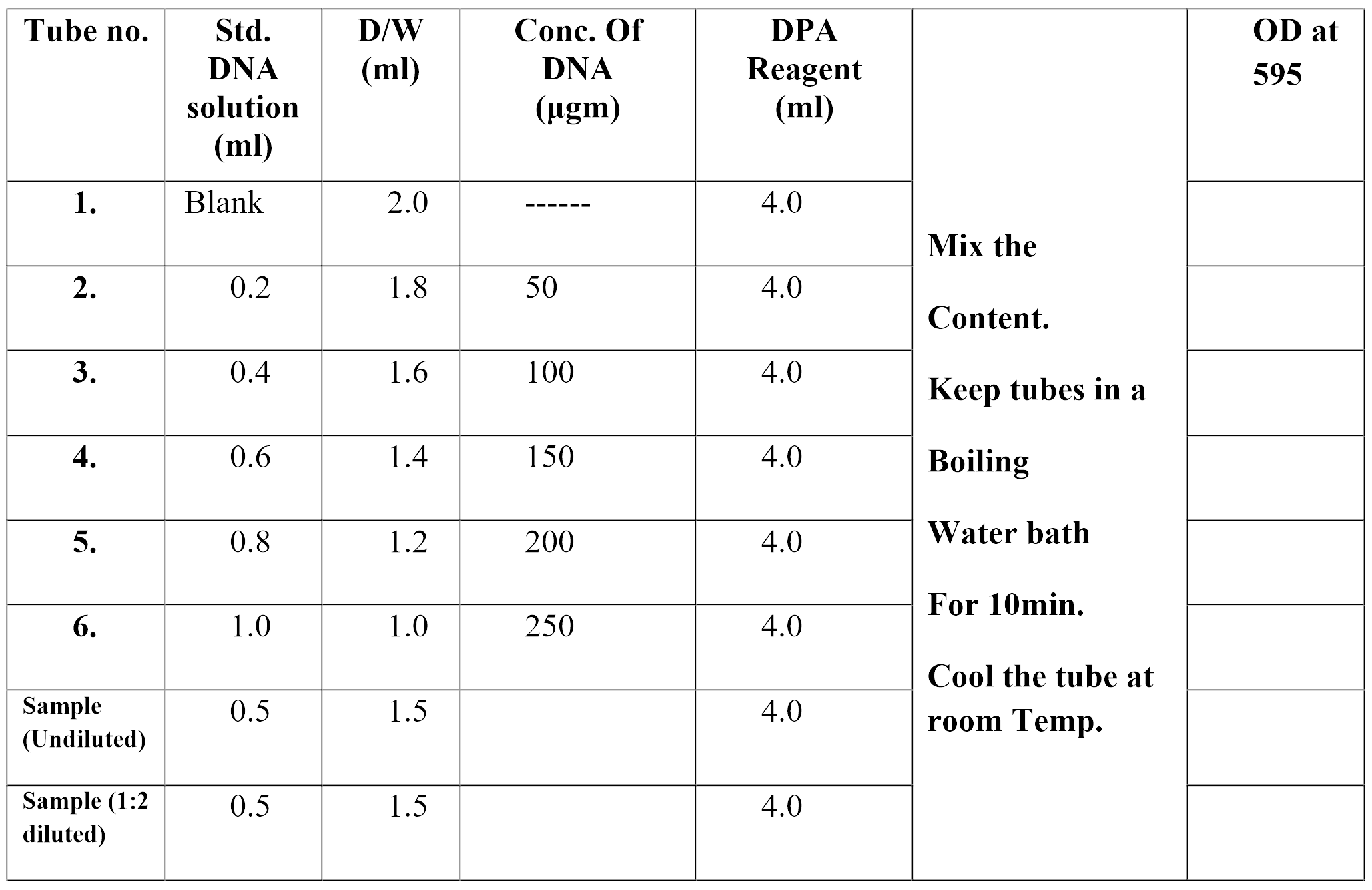
Take O.D. at 595nm and calculate the DNA concentration from
standard graph of sample.
Reference
Burton, K. (1956). A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochemical journal, 62(2), 315