What is chitin?
Chitin is a complex homopolysaccharide consisting of units
of amino sugar glucosamine that accounts for the second most abundant
polysaccharide of nature after cellulose.
- It
is widely distributed in nature, found in the cell walls of fungi,
the exoskeleton of arthropods, and certain structures of other invertebrates.
- Chitin,
like cellulose, doesn’t accumulate in the biosphere as a result of the
extensive hydrolytic activity of soil
microorganisms.
- Chitin
is associated covalently or non-covalently with other structural molecules
as well as the environment.
- The
deacetylated derivative of chitin called chitosan has biomedical
applications as it acts as an antimicrobial and hydrating agent. The
conversion of chitin to chitosan is catalyzed either by enzymatic or
chemical hydrolysis.
- The
term ‘chitin’ is derived from the Greek word ‘chiton’ which means a coat
of mail.
- Depending
on the source, chitin occurs in two forms; α and β conformation. A third
less discussed γ form is also known.
- These
allomorphs differ from one another in their orientation of the
micro-fibrils.
- Chitin
is considered an essential polymeric structure due to its characteristics
like high porosity, biodegradability, predictable degradation rate, and
structural integrity.
- Chitin
is similar to cellulose in that it is also indigestible by vertebrate
animals due to the lack of enzyme system required for its degradation.
Structure of chitin
Chitin is a β(1,4)-linked homopolymer of N-acetyl
glucosamine derivative of glucose, and it shares close structural similarity to
cellulose.
- In
chitin, the alcoholic OH group of the second carbon atom of β-D-glucose
units is replaced by an N-acetylamino group.
- Chitin
is insoluble in both aqueous and non-polar solvent despite the presence of
charges at the acetyl groups.
- Chitin
exists as a linear polymer of N-acetyl-D-glucosamine units linked together
by β-1,4-glucosidic linkages. This structure results in a
three-dimensional α-helix configuration.
- The
stability of the α-helix chitin structure is brought about by the hydrogen
bonding of the N-acetyl side chains.
- In
nature, however, the chitin polymers bind extracellularly by
intermolecular hydrogen bonding that forms a crystalline microfibril
structure.
On the basis of the direction of the chitin fibers and bonds
within the polymer, chitin exists in three different conformations.
α-chitin
- In
α-chitin, the chitin fibers are antiparallel, resulting in an orthorhombic
orientation.
- Strong
hydrogen bonds stabilize this conformation of chitin structure in the a
and b direction while the forces in the c direction are weak.
- Two
different types of hydrogen bonding are present in the α-chitin that
stabilizes the crystalline structure; intrasheet and intersheet hydrogen
bonds.
- The
intrasheet hydrogen bond occurs between the carbonyl group of amide I and
amide II.
- The
intersheet hydrogen bonds exist between the CH2OH side chain
and the carbonyl group.
- The
decomposition temperature of the crystalline structure of the chitin
depends on the hydrogen bonding and thus it is highest at 330°C in
α-chitin.
β-chitin
- In
β-chitin, the chitin fibers are parallel to each other, stabilized only by
the intrasheet hydrogen bonding. It consists of monoclinic units.
- The
decomposition temperature of the crystalline structure of β-chitin is the
lowest at 230°C as it has the lowest number of hydrogen bonds.
γ-chitin
- The
γ-chitin conformation is characterized by alternating parallel and
antiparallel aligned chitin fibers.
- The
number and direction of hydrogen bonding in γ-chitin is similar to that in
α-chitin. Both intersheet and intrasheet hydrogen bonding exists in
γ-chitin.
- The
decomposition temperature of the crystalline structure of γ-chitin is
310°C.
What are Chitinases?
- Chitinases
are a group of glycosyl hydrolases that range in size from 20kDa to 90kDa
and are found in a wide range of organisms like bacteria, fungi, yeasts,
plants, actinomycetes, and animals.
- Chitinases
degrade chitin directly into smaller, low molecular weight chitooligomers,
which serve industrial, agricultural, and medical functions.
- Chitinases
works by degrading the β-1,4-linkages that exist between N-acetyl
glucosamine units to reduce the length of the polymer, ultimately leading
to the formation of monomeric units.
- Chitinases
are broadly divided into two groups; exo-chitinases and endo-chitinases.
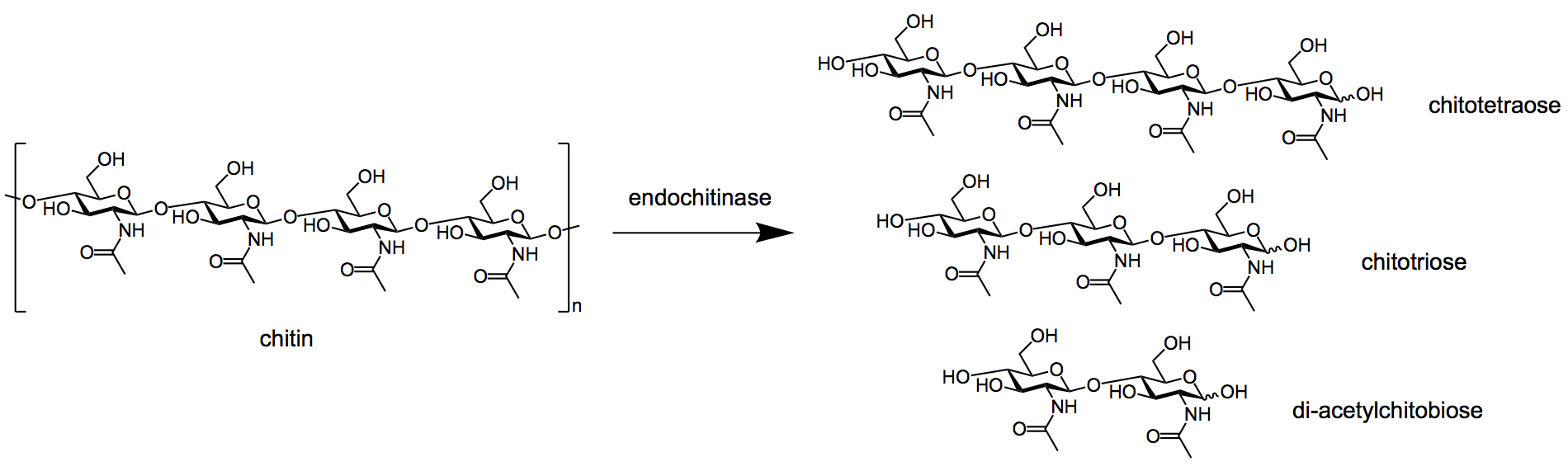
- Endochitinases
split the polymeric chains at random sites internally, thereby forming the
dimeric units of di-cetylchitobiose and soluble low molecular mass
multimers of glucosamine as chitotriose and chitotetraose.
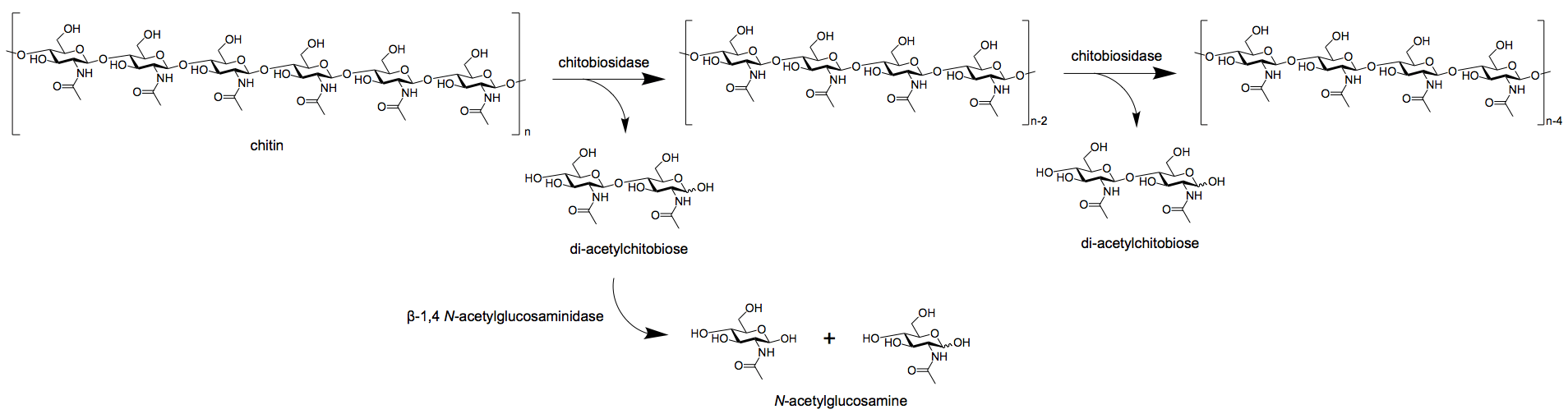
- Exochitinases,
in turn, are further divided into two groups; chitobiosidases and
1,4-β-glucosaminidases.
- Chitobiosidases
catalyze the subsequent release of di-acetylchitobiose beginning at the
non-reducing of the chitin fibrils.
- 1,4-β-glucosaminidases
cleave the oligomeric products of endochitinase and chitobiosidases to
produce monomeric products of glucosamine.
- Five
distinct classes and three families (Family 18, 19, and 20) of chitinases
have been proposed based on the amino acid similarity between chitinases
from various organisms.
- Family
18 chitinases are mostly sourced from viruses, fungi, bacteria, and some
plants. These contain a number of conserved repeats of amino acids and
enzyme core with 8 strands of parallel β-sheets.
- Family
19 is of plant chitinases and Treptomyces Family 18 and
19 chitinases do not share amino acid similarity and have a different
three-dimensional structure.
- Family
20 contains N-acetylglucosaminidases from bacteria, fungi, and humans.
- Chitinases
consist of a diverse group of enzymes that are different in their
molecular structure, substrate specificity, and catalytic mechanism.
- Due
to their industrial applications, the production of microbial chitinases
has increased over the years.
- Methods
like fed-batch fermentation, continuous fermentation, and liquid batch
fermentation are employed.
Microorganisms involved in chitin degradation
Chitinolytic bacteria
- Chitinolytic
bacteria are widely distributed in different habitats, and chitinases are
produced by many genera of Gram-negative and Gram-positive bacteria, but
not by Archaebacteria.
- Chitinolytic
bacteria of the genera Vibrio and Photobacterium are
associated with zooplankton and particulate matter that are found in a sea
associated with zooplanktons and carapaces.
- Bacterial
species of Vibrio, Photobacterium, Aeromonas, Cytophaga,
Streptomyces, Photobacterium, Bacillus, Clostridium, and Chromobacterium are
well-known chitinolytic bacteria.
- These
are specialized utilizers of diacetylchitobiose, and the accumulation of
N-acetylglucosamine indicates non-utilizable monomers during random
hydrolysis of chitin oligomers.
- Chitinolytic
bacteria are also abundant in freshwaters, characteristic genera of Serratia,
Chromobacterium, Pseudomonas, Flavobacterium, and Bacillus,
with Cytophaga johnsonae.
Chitinolytic fungi
- The
primary habitat of chitinolytic fungi is the soil where the chitinolytic
activity of fungi might even exceed that of bacteria.
- The
most common fungal species involved in chitinolysis include Mucorales
like Mortierella spp, and Deuteromycetes and Ascomycetes
like Aspergillus, Verticillium, Thielavia,
Trichoderma, Penicillium, and Humicola.
- The
chitinolytic system in these fungi is inducible, and the activity
increases with the increase in the chitin-rich substrate.
- Freshwater
species Chrytriomyces and Karlingia are
obligate chitinophile that degrade chitin to fulfill their nutritional
requirement.
Slime mold, protozoa, and algae
- Myxomycetes
(true slime molds) like Physarum polycephalum are a rich
source of lytic enzymes that produce a complex of extracellular
chitinases.
- Soil
protozoa like Hartmanella and Schizopyrenus,
along with slime mold Plasmodium are also known to
produce chitinases that participate in the digestion of chitinous food
particles engulfed by these invertebrates.
- A
colorless heterotrophic diatom, Nitzchia alba, is
the only known diatom to digest chitin.
Enzymes involved in the degradation of chitin
There are different classes and families of chitinases that
act on different stages of chitin degradation and might even utilize different
mechanisms of degradation. There important families of chitinases include
family 18, 19, and 20 chitinases. These chitinases might differ in their source
and their structural components.
Family 18 chitinases
- Family
18 chitinases are retaining enzymes that include both chitinases as well
as chitosanases.
- These
are found in many organisms, including archaea, bacteria, eukaryote, and
viruses. This family of enzymes is widely studied.
- These
chitinases are further classified into subclass A, B, and C on the basis
of their amino acid sequence similarities.
- In
some of the chitinases of the family 18, only a catalytic domain is found,
whereas others might have one or more carbohydrate-binding modules.
- The
mechanism of catalysis in family 18 chitinases has some modifications
compared to the typical double retaining mechanism.
- Instead
of using a carboxylate side chain of the enzyme as the catalytic
nucleophile, family 18 chitinases use the acetamido group of the C-1
sugar.
Family 19 chitinases
- Family
19 chitinases are different from family 18 chitinases as they use an
inverting mechanism leading to α-anomeric hydrolysis rather than the
retaining mechanism.
- Traditionally,
family 19 chitinases were known to exist only in plants, but some
bacterial chitinases are also added to the family over the years.
- The
family 19 chitinases consist of catalytic domains that have a
lysozyme-like fold with shallow substrate-binding grooves that are not
rich in aromatic residues.
- Due
to the lack of information about the structure of these enzymes,
information on their interaction with the substrate is also limited.
Chitin deacetylases
- Chitin
deacetylases include enzymes like peptidoglycan N-acetyl glucosamine
deacetylase and peptidoglycan N-acetylmuramic acid deacetylase that
removes the acetyl groups in the substrates.
- These
enzymes are essential as they reduce the branching in the structure, which
reduces the steric hindrance for other exo and endoenzymes.
Factors affecting chitin degradation
Chitin degradation in soil or on artificial media can be
affected by several factors, some of which are:
Moisture content
- The
process of chitin degradation occurs rapidly in the presence of free water
and complete saturation.
- However,
the increase in the amount of water has a minimal effect on the
degradation process until aeration becomes impaired due to logging.
Added glucose
- The
addition of glucose in the media or soil decreases the rate of chitin
degradation as the organisms tend to utilize the readily available source
rather than chitin.
- Glucose
is a ready energy source which is easy to metabolize. This, in turn,
causes a delay or decreased chitin degradation.
- In
the absence of these sources, however, chitin degradation enhances.
Aeration
- Since
most of the chitinolytic microorganisms are aerobic and thrive in
high-oxygen environments, the rate of chitin degradation also increases.
- Some
amount of degradation can also be observed in some concentration of CO2 as
it allows facultative aerobes and anaerobes to be involved.
- Pure
oxygen environment might be toxic in some cases, especially when readily
energy source is available.
Organic matter
- The
presence of organic matter rich in chitin also increases the rate of
chitin degradation.
- The
increase in organic matter increases the substrate concentration. The rate
of degradation might be slow at first as the microorganisms utilize more
readily available energy forms, followed by chitin degradation.
- Other
forms of energy like cellulose and lignin should also be present as it
allows the growth of microorganisms for the formation of proteins and
enzymes.
Process of chitin degradation
The hydrolysis of chitin occurs in a two-step process;
Depolymerization
- Depolymerization
is the process of reduction of chitin polymer length by the breakdown of
β-1,4 linkages between the N-acetyl glucosamine units.
- This
process results in the release of N-acetylglucosamine units by the action
of chitinases of chitosanases.
- The
initial enzymatic action is of chitinases which are either endochitinases
or exochitinases, resulting in the formation of chitobioses or
chitotrioses.
- The
chitobioses are further acted upon by exoenzymes like chitobioases to form
monomeric units.
- In
some cases, chitin might be converted into chitosan, which requires the
action of chitsonases.
Deacetylation
- Depolymerization
is followed by acetylation which causes the release of glucosamine units
and acetic acid.
- Chitin
deacetylases act on the N-acetyl glucosamine dimer or trimers, resulting
in the catalytic degradation of the larger molecule into smaller ones.
- The
end products of this step are glucosamine and acetic acid, which are then
utilized by the microorganism for various purposes.
Mechanisms of microbial degradation of chitin
- The
vast amount of chitin produced by different sources is balanced by an
equal rate of recycling of the substrate.
- Most
of the chitin degradation occurring in nature is microbial, carried out by
a different group of microorganisms.
- Chitin
degradation occurs in different habitats like the sea, animal guts, and
the soil.
- Microbial
chitin degradation occurs by one of the two mechanisms; chitinoclastic
mechanism and deacetylation mechanism.
Chitinoclastic
- Chitinoclastic
mechanism of chitin degradation occurs solely by the hydrolysis of
glycosidic bonds. Organisms that degrade chitin by this mechanism are
called chitinolytic organisms.
- In
this mechanism, the substrate is acted upon by the chitinolytic system,
consisting of chitinases.
- Exochitinase
breakdown acetylchitobiose units from the non-reducing end of the
polysaccharide chain.
- Endochitinase
cleaves glycosidic linkages randomly along the chain, eventually resulting
in the formation of diacetylchitibiose as the major product, along with
some tri-acetyl chitotriose.
- The
activities of these enzymes may not always be distributed, as the action
of these enzymes is dependent on the nature of the substrate.
- Chitobiose
(structurally, diacetylchitobiose) is hydrolyzed to N-acetylglucosamine by
β-N-acetylglucosaminidase.
- In
some cases, β-N-acetylglucosaminidases might also act weakly as
exochitinases, cleaving monosaccharide units from the non-reducing ends of
the polymeric chains.
- Together,
the chitinases and β-N-acetylglucosaminidases form ‘the chitinolytic
system’.
Deacetylation
- Deacetylation
is an alternate mechanism of chitin degradation which involves the
conversion of chitin into chitosan.
- This
mechanism of chitin degradation is important in the freshwater system or
soil sediments.
- The
group of enzymes involved in the deacetylation mechanism is termed
deacetylases. These enzymes catalyze the process of deacetylation of
N-acetylglucosamine polymer.
- The
hydrolysis of chitosan occurs in the presence of chitosanases that
breakdown the linkages between the β-glucosamine units linked together by
β-1,4-glycosidic linkages.
- This cleavage results in the release of chitobiose (glucosaminyl-(1-4)- β-glucosaminide) which is then further degraded by glucosoaminidase to obtain glucosamine units.