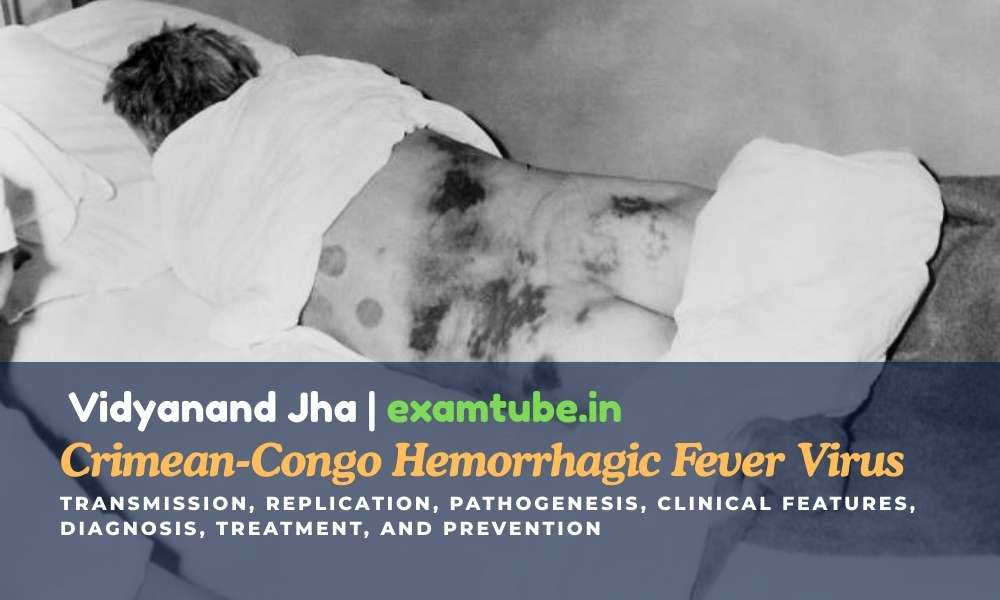
Structure of Crimean-Congo Hemorrhagic Fever Virus
- Crimean-Congo
hemorrhagic fever virus (– causative agent of CCHF) falls under
the family Bunyaviridae (– family of segmented RNA viruses)
and genus Nairovirus (– tick-borne bunyaviruses).
- They
are spherical particles (– round virus particles) measuring 90
to 120 nm (– nanometer size range) in diameter with 5–10 nm
projections (– surface spikes) visible on the surface.
- They
are an enveloped virus (– surrounded by lipid membrane)
which comprises two glycoproteins (G1 and G2) (– viral surface
proteins involved in attachment and fusion).
- The
genome is tripartite (– divided into three segments), negative-sense
RNA (– RNA complementary to mRNA), termed the large (L),
medium (M), and small (S) segments, which are associated with protein
to form nucleocapsids (– RNA–protein complexes).
- The nucleocapsid
(– viral genome plus proteins) is surrounded by a lipid-containing
envelope (– outer viral membrane).
- The
nucleocapsids include the RNA-dependent RNA polymerase (L protein)
(– enzyme that synthesizes viral RNA).
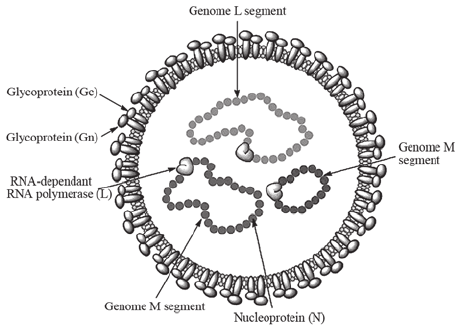
Figure: Crimean-Congo hemorrhagic fever virus structure.
Source: DOI: 10.3892/br.2015.545
Genome of Crimean-Congo Hemorrhagic Fever Virus
- The
genome is linear, tripartite, segmented negative-stranded
RNA (– linear RNA genome with three segments of negative polarity).
- It
comprises three segments (– genome divisions): large (L),
medium (M), and small (S).
- The L
segment is 12,164 nucleotides, the M segment is 4,888
nucleotides, and the S segment is 1,712 nucleotides (–
segment lengths).
- It
encodes four to six proteins (– viral structural and functional
proteins).
- Terminal
complementary nucleotide sequences (– matching RNA ends) are
conserved on the L, M, and S segments (– conserved genomic
regions).
- The S
segment of nairoviruses encodes only a large N protein (–
nucleoprotein that binds RNA) and has no known nonstructural
protein coding information (– lacks accessory proteins).
- The M
segment of the nairovirus seems to encode only G2 and G1 (–
envelope glycoproteins).
- Nucleotide
sequencing (– determining RNA sequence) of the L RNA of
Crimean-Congo hemorrhagic fever virus revealed that the segment is 12,164
nucleotides in length and encodes 3,944 amino acids (–
protein length).
- The viral
RNA-dependent RNA polymerase (L) (– viral transcription enzyme)
binds to a promoter (– transcription initiation site) on
each encapsidated segment (– RNA coated with protein) and transcribes
the mRNA (– messenger RNA synthesis).
- These
mRNAs are capped (– addition of 5′ cap) by the L protein
during synthesis using cap-snatching (– stealing caps from host
mRNA).
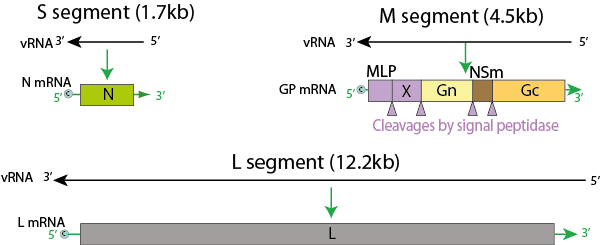
Figure: Genome of Nairovirus, Source: Viral Zone
Epidemiology of Crimean-Congo Hemorrhagic Fever Virus
- Crimean-Congo
hemorrhagic fever (CCHF) virus, of the Nairovirus genus (–
tick-borne virus group), was first recognized in the Crimean
peninsula (– region in southern Ukraine) during an outbreak of hemorrhagic
fever (– bleeding fever) among agricultural workers (–
occupational exposure).
- The
same virus was isolated in 1956 from a single patient in the
present-day Democratic Republic of Congo, leading to the actual naming
(– Crimean + Congo).
- Although
animals and humans (– multiple hosts) can be infected, only
the latter (– humans) develop the disease.
- Crimean-Congo
hemorrhagic fever is a tick-transmitted viral disease (–
arthropod-borne infection) found in Bulgaria, Yugoslavia, the
former Soviet Union, China, Iraq, United Arab Emirates, Pakistan, and
sub-Saharan Africa.
- The vector
tick (– transmitting arthropod) is usually of the Hyalomma
(– hard tick genus).
Source: CDC, 2014
Transmission of Crimean-Congo Hemorrhagic Fever Virus
- Ixodid
ticks (– blood-sucking arthropods) especially those of the
genus Hyalomma (– tick genus), are both a reservoir (–
organism that harbors virus) and a vector (– transmitter of
virus) for the CCHF virus (– Crimean-Congo hemorrhagic fever
virus).
- Transmission
to humans occurs through contact with infected ticks or animal
blood (– viremic livestock blood).
- CCHF
can be transmitted from one infected human to another by contact with infectious
blood or body fluids (– secretions capable of transmission).
- Improper
sterilization of medical equipment, reuse of injection needles,
and contamination of medical supplies (– hospital-acquired
exposure) can result in spread of CCHF in hospital premises.
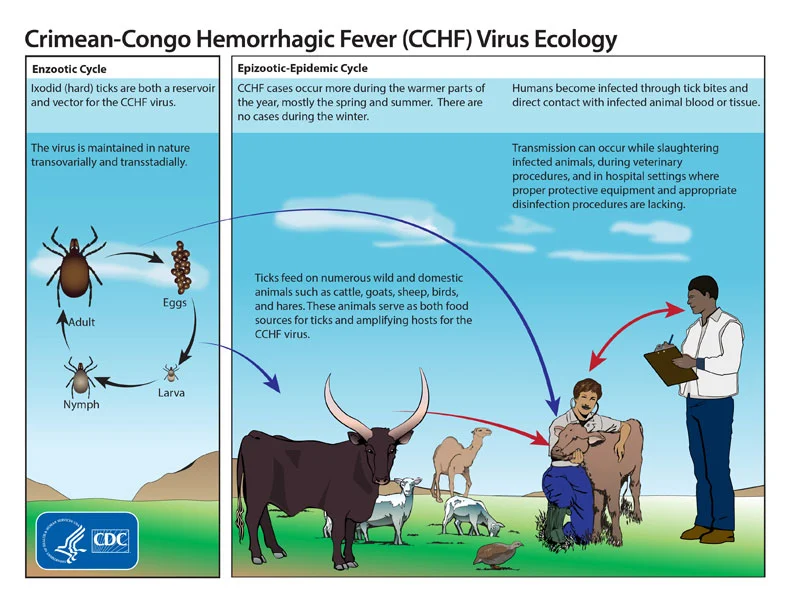
Figure: Life cycle of the Crimean-Congo hemorrhagic fever
virus, Source: CDC
Replication of Crimean-Congo Hemorrhagic Fever Virus
- Virus
attaches to host receptors (– cell surface binding sites)
through glycoprotein (– viral attachment protein) and is endocytosed
(– internalized) into vesicles in the host cell.
- Fusion
of virus membrane with the vesicle membrane results in the
release of the ribonucleocapsid (– RNA–protein complex) into
the cytoplasm (– intracellular fluid).
- The RNA-dependent
RNA polymerase (RdRp) (– enzyme synthesizing RNA from RNA)
complex initiates transcription (– viral mRNA synthesis) by
binding to the leader sequence (– regulatory RNA region) at
the 3′ end of the genomic negative-strand RNA, and viral mRNAs
(– messenger RNAs) are capped in the cytoplasm.
- During
replication (– genome copying), the RNA-dependent RNA
polymerase complex binds to the leader sequence on the encapsidated
(-)RNA genome (– protein-coated RNA) and starts replication.
- The antigenome
(– complementary RNA strand) is concomitantly encapsidated (–
coated with nucleoproteins) during replication and replicates to give
rise to negative-sense genome (– infectious RNA strand).
- Nucleocapsids
(– RNA-protein assemblies) assembled induce formation of membrane
curvature (– bending of host membrane) in the host cell
membrane and wrap up in the forming bud at the Golgi apparatus (–
cellular protein-sorting organelle), releasing the enveloped virion
(– lipid-coated virus) by exocytosis (– vesicle-mediated
release).
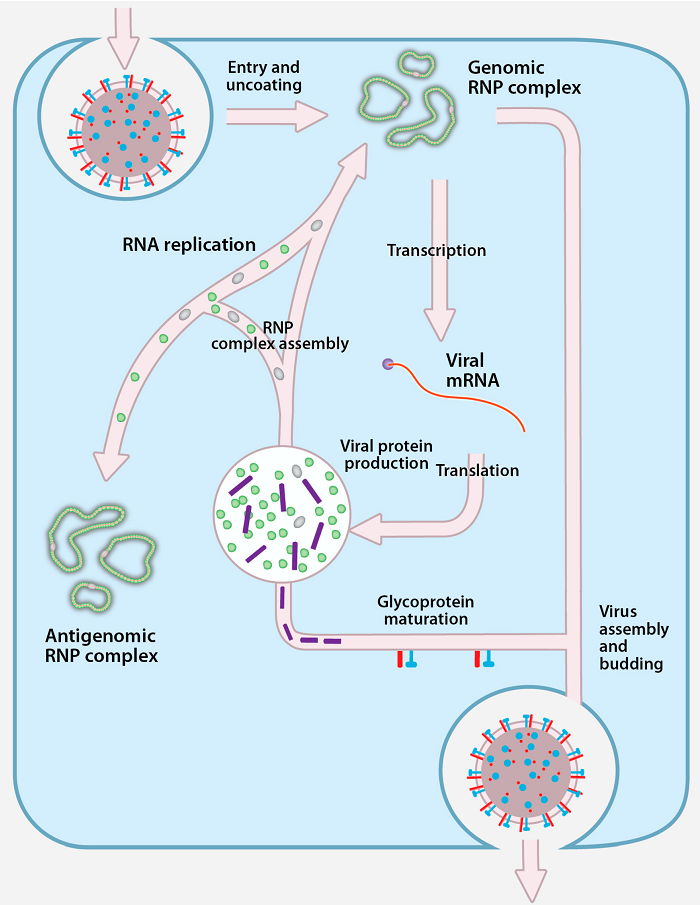
Figure: Replication of Crimean-Congo Hemorrhagic Fever
Virus, Source: doi:10.3390/v8040106
Pathogenesis of Crimean-Congo Hemorrhagic Fever
- The gut
of the vector (– tick digestive tract) is infected initially,
and after a few days or weeks the virus appears in the saliva (–
infectious secretions).
- When
the vector takes a blood meal (– feeding on host blood), the
infective saliva enters the small capillaries (– tiny
blood vessels) or lymphatics (– lymph vessels) of the
human or other vertebrate host (– animal with backbone).
- An incubation
period (– time before symptoms) of a few days ensues, after
which the vertebrate host develops viremia (– virus present in
blood).
- The
host becomes febrile (– develops fever), manifesting the
more serious signs and symptoms characteristic of the infecting virus.
- A typical humoral immune response (– antibody-mediated immunity) leads to cessation of viremia and clinical recovery in most cases, with immunoglobulin M (IgM) (– early antibody) predominating initially, followed by immunoglobulin G (IgG) (– long-term antibody), and the host recovers unless a specific target organ (– organ preferentially damaged) is affected.
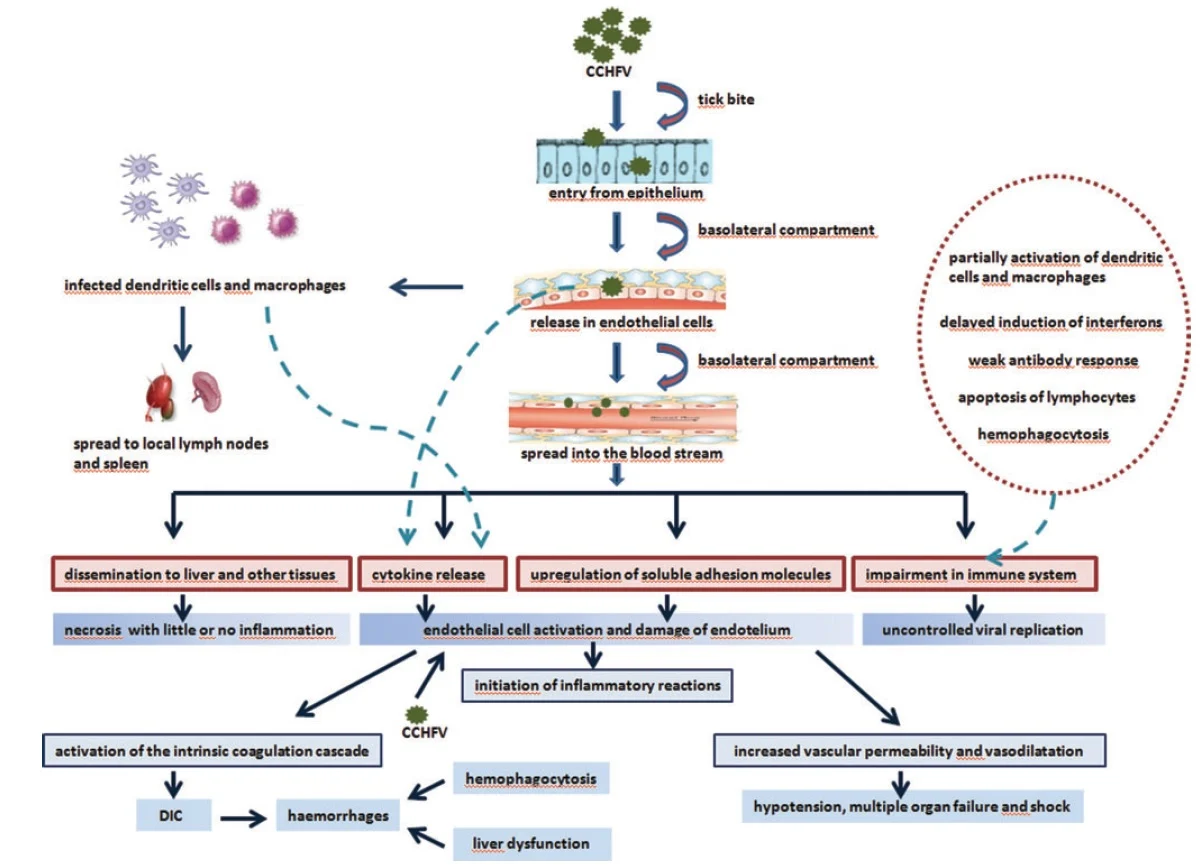
- The
target organ is the liver (– metabolic organ) and vascular
endothelium (– inner lining of blood vessels) in Crimean-Congo
hemorrhagic fever.
- This
further leads to hemostatic failure (– inability to stop
bleeding) by stimulating platelet aggregation and degranulation
(– platelet activation), with subsequent activation of the intrinsic
coagulation cascade (– clotting pathway).
- Proinflammatory
cytokines (– immune signaling molecules) are key regulators in
the pathogenesis and mortality of patients with CCHF.
- Levels
of Interleukin-6 (IL-6) (– inflammatory cytokine) and Tumor
Necrosis Factor-α (TNF-α) (– major inflammatory mediator) are
significantly higher in patients with fatal CCHF.
Clinical Manifestations of Crimean-Congo Hemorrhagic Fever
- Following
an incubation period of 3–21 days, a non-specific febrile
illness (– fever with general symptoms) of abrupt onset
develops.
- Initial
signs and symptoms include headache, high fever, back
pain, joint pain, stomach pain, nausea, and non-bloody
diarrhea.
- Red
eyes, flushed face, red throat, and petechiae (–
small red hemorrhagic spots) on the palate are common.
- This
is accompanied by hypotension (– low blood pressure), relative
bradycardia (– slow heart rate), tachypnea (– rapid
breathing), conjunctivitis (– eye inflammation), pharyngitis
(– throat inflammation), and cutaneous flushing or rash (–
skin redness).
- Symptoms
may also include jaundice (– yellowing due to liver damage),
and in severe cases, changes in mood and sensory perception
(– neurological involvement).
- The hemorrhagic phase (– bleeding stage) is generally short and has a rapid course with signs of progressive hemorrhage and diathesis (– bleeding tendency) including petechiae, conjunctival hemorrhage, epistaxis (– nose bleed), hematemesis (– blood in vomit), hemoptysis (– coughing blood), and melena (– black stools).
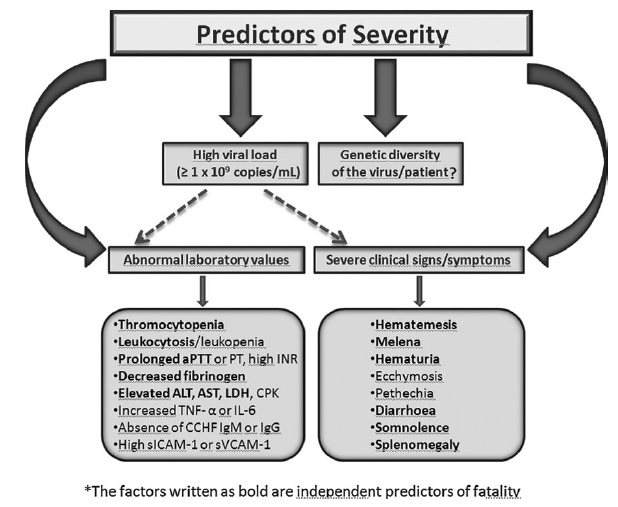
- Internal
bleeding, including retroperitoneal (– abdominal cavity)
and intracranial hemorrhage (– bleeding in brain), may
occur.
- Hepatosplenomegaly
(– enlarged liver and spleen) may be present, and in severe cases
death occurs due to multiorgan failure, disseminated
intravascular coagulation (DIC) (– widespread clotting), and circulatory
shock (– failure of blood circulation).
Diagnosis of Crimean-Congo Hemorrhagic Fever Virus
- Virus
isolation by intracranial inoculation of suckling mice (–
sensitive animal model) is thought to be the most sensitive system
available; however, several sensitive cell culture systems (–
virus-growing cells) such as Vero, LLC-MK2, and BHK-21
cell lines are available.
- Immunohistochemical
staining (– antibody-based tissue staining) can show evidence
of viral antigen in formalin-fixed tissues (– preserved
specimens).
- Detection
of antibodies (IgG and IgM) (– immune response markers) by ELISA
(– enzyme-linked immunosorbent assay).
- Detection
of viral antigen, viral RNA sequence by RT-PCR (–
molecular RNA detection), in blood or tissues collected from a fatal
case, and virus isolation.
Treatment of Crimean-Congo Hemorrhagic Fever
- Supportive
treatment (– symptom-based care) including fluid balance,
correction of electrolyte abnormalities, oxygenation, and hemodynamic
support (– blood pressure maintenance).
- The
virus is sensitive in vitro (– laboratory conditions) to the
antiviral drug ribavirin, which is administered in both IV and
oral forms (– injection and tablets) with apparent benefit.
Prevention and Control of Crimean-Congo Hemorrhagic Fever Virus
- There
is no safe and effective vaccine currently available for human use.
- In
case of known direct contact with blood or secretions (–
exposure risk) of a probable or confirmed case, such as needlestick
injury (– sharp injury) or contact with mucous membranes
(– eye or mouth), baseline blood studies should be carried out and oral
ribavirin started as post-exposure prophylaxis (– prevention
after exposure).
- Use
of insect repellent on exposed skin and clothing.
- Wearing
gloves and other protective clothing (– barrier protection).
- Avoiding
contact with blood and body fluids of livestock or humans showing
symptoms of infection.
- Use of proper infection control precautions (– hospital safety measures) to prevent occupational exposure (– workplace infection risk).