Chromatography
Chromatography
is an important biophysical technique that enables the separation, identification,
and purification of the components of a mixture for qualitative and
quantitative analysis.
- A wide range of chromatographic procedures makes
use of differences in size, binding affinities, charge, and other
properties to separate materials.
- It is a powerful separation tool that is used in
all branches of science and is often the only means of separating
components from complex mixtures.
- Chromatography is a very useful technique as it
allows the separation of components of a mixture on the basis of their nature,
structure, size, and other properties.
- Chromatography, in general, is based on the
principle that components of a mixture are separated when the mixture
added to a mobile phase is moved through a stationary phase (which mostly
is a solid surface), resulting in some components of the mixture being
attached to the stationary phase. At the same time, the rest is passed
along with the mobile phase.
- Thus, there are two essential components of all
chromatography techniques.
What is a stationary phase?
The
stationary phase in chromatography is the phase that is either a solid or
liquid particle attached to a glass or a metal surface on which the components
of the mixture to be separated is absorbed selectively.
- The term stationary refers to the fact that this
phase remains stationary while the other phase moves.
- Most substances used as stationary phases are
porous, thus allowing the attachment of components during chromatography.
- The stationary phase to be selected for a
chromatographic process depends on the nature of the components to be
separated and the type of chromatography.
- Depending on the type of chromatography gel beads, thin uniform paper, silica, glass, some gases, or even liquid components are used as a stationary phase.
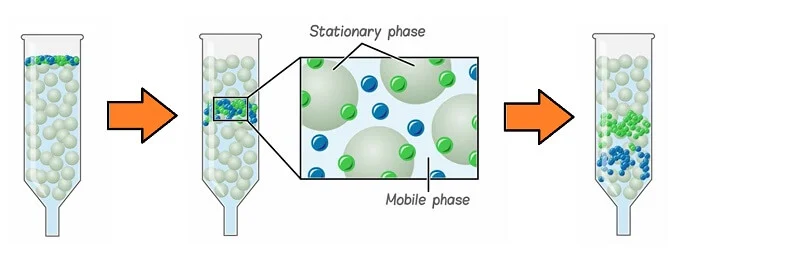
What is the mobile phase?
The mobile
phase in chromatography is the phase that is either liquid or gas that is
passed through a chromatographic system where the components of the mixture are
separated at different raters by adsorbing them to the stationary phase.
- The mobile phase is the solvent that carries the
mixture as it moves down the stationary phase.
- The term mobile indicates that the phase is moving
down the chromatographic system, whereas the other phase remains
stationary.
- Substances used as mobile phases are selected for a
chromatographic process depending on the nature of the components to be
separated and the type of chromatography.
- Alcohol, water, acetic acid, acetone, or some gases are the commonly used mobile phase in different chromatographic techniques.
Types of Chromatography
1. Affinity chromatography
Affinity
chromatography is a separation technique where the components of a mixture are
separated based on their affinity towards the stationary phase of the system.
Principle
of Affinity chromatography
- This chromatography technique is based on the
principle that components of a mixture are separated when the element
having an affinity towards the stationary phase binds to the stationary
phase. In contrast, other components are eluted with the mobile phase.
- The substrate/ ligand is bound to the stationary
phase so that the reactive sites for the binding of components are
exposed.
- Now, the mixture is passed through the mobile phase
where the components with binding sites for the substrate bind to the
substrate on the stationary phase while the rest of the components are
eluted out with the mobile phase.
- The components attached to the stationary phase are
then eluted by changing the pH, ionic strength, or other conditions.

Steps of Affinity chromatography
- The column is prepared by loading it with solid
support like agarose or cellulose, onto which the substrate/ ligand with
the spacer arm, is attached.
- The mobile phase containing the mixture is poured
into the column at a constant rate.
- Once the process is complete, the ligand-molecule
complex is eluted from the stationary phase by changing the conditions
that favor the separation of ligand and components of the mixture.
Uses of Affinity chromatography
- Affinity chromatography is used as a staple
separation technique from enzymes and other proteins.
- This principle is also applied in the in vitro
antigen-antibody reactions.
- This technique is used for the separation of
components as well as the removal of impurities from a mixture.
- Affinity chromatography can be used in the
detection of mutation and nucleotide polymorphisms in nucleic acids.
Examples of Affinity chromatography
- The purification of coli β-galactosidase from a
mixture of proteins using the p-aminophenyl-1-thio-β-D-galactopyranosyl
agarose as the affinity matrix.
- The removal of excess albumin and α2-macroglobulin
from the serum albumin.
2. Anion exchange chromatography
Anion
exchange chromatography is the separation technique for negatively charged
molecules by their interaction with the positively charged stationary phase in
the form of ion-exchange resin.
Principle of Anion exchange chromatography
- This technique is based on the principle of
attraction of positively charged resin and the negatively charged analyte.
Here the exchange of positively charged ions takes place to remove the
negatively charged molecules.
- The stationary phase is first coated with positive
charges where the components of the mixture with negative charges will
bind.
- An anion exchange resin with a higher affinity to
the negatively charged components then binds the components, displacing
the positively charged resin.
- The anion exchange resin-component complex then is
removed by using different buffers.

Steps of Anion exchange chromatography
- A column packed with positively charged resin is
taken as the stationary phase.
- The mixture with the charged particles is then
passed down the column where the negatively charged molecules bind to the
positively charged resins.
- The anion exchange resin is then passed through the
column where the negatively charged molecules now bind to the anion
exchange resin displacing the positively charged resin.
- Now an appropriate buffer is applied to the column
to separate the complex of anion exchange resins and the charged
molecules.
Uses of Anion exchange chromatography
- Anion exchange chromatography is used to separate
proteins and amino acids from their mixtures.
- Negatively charged nucleic acids can be separated,
which helps in further analysis of the nucleic acids.
- This method can also be used for water purification
where the anions are exchanged for hydroxyl ions.
- Anion exchange resins can be used for the
separation of metals as they usually have negatively charged complexes
that are bound to the anion exchangers.
Examples of Anion exchange chromatography
- The separation of nucleic acids from a mixture
obtained after cell destruction.
- The separation of proteins from the crude mixture
obtained from the blood serum.
3. Cation exchange chromatography
Anion
exchange chromatography is the separation technique for positively charged
molecules by their interaction with negatively charged stationary phase in the
form of ion-exchange resin.
Principle of Cation exchange chromatography
- This technique is based on the principle of
attraction of negatively charged resin and the positively charged analyte.
Here the exchange of negatively charged ions takes place to remove the
positively charged molecules.
- The stationary phase is first coated with negative
charges where the components of the mixture with positive charges will
bind.
- A cation exchange resin with a higher affinity to
the positively charged components then binds the components, displacing
the negatively charged resin.
- The cation exchange resin-component complex then is
removed by using different buffers.
Steps of Cation exchange chromatography
- A column packed with negatively charged resin is
taken as the stationary phase.
- The mixture with the charged particles is then
passed down the column where the positively charged molecules bind to the
negatively charged resins.
- The cation exchange resin is then passed through
the column where the positively charged molecules now bind to the cation
exchange resin displacing the negatively charged resin.
- Now an appropriate buffer is applied to the column
to separate the complex of cation exchange resins and the charged
molecules.
Uses of Cation exchange chromatography
- Cation exchange chromatography is used for the
analysis of the products obtained after the hydrolysis of nucleic acids.
- This can also be used for the separation of metals
where the metal ions themselves bind to the negatively charged resins to
remove the negatively charged complexes.
- Cation exchange chromatography helps in purification
of water by exchanging the positively charged ion by the hydrogen ions.
- It is also used to analyze the rocks and other
inorganic molecules.
Examples of Cation exchange chromatography
- The separation of positively charged lanthanoid
ions obtained from the earth’s crust.
- The determination of total dissolved salts in
natural waters by analyzing the presence of calcium ions.
4. Column chromatography
Column
chromatography is the separation technique where the components in a mixture
are separated on the basis of their differential adsorption with the stationary
phase, resulting in them moving at different speeds when passed through a
column.
It is a solid-liquid
chromatography technique in which the stationary phase is a solid & mobile
phase is a liquid or gas.
Principle of Column chromatography
- This technique is based on the principle of
differential adsorption where different molecules in a mixture have
different affinities with the absorbent present on the stationary phase.
- The molecules having higher affinity remain
adsorbed for a longer time decreasing their speed of movement through the
column.
- However, the molecules with lower affinity move
with a faster movement, thus allowing the molecules to be separated in
different fractions.
- Here, the stationary phase in the column
chromatography also termed the absorbent, is a solid (mostly silica) and
the mobile phase is a liquid that allows the molecules to move through the
column smoothly.

Steps of
Column chromatography
- The column is prepared by taking a glass tube that
is dried and coated with a thin, uniform layer of stationary phase
(cellulose, silica).
- Then the sample is prepared by adding the mixture
to the mobile phase. The sample is introduced into the column from the top
and is allowed to pass the sample under the influence of gravity.
- The molecules bound to the column are separated by
elution technique where either solution of the same polarity is used
(isocratic technique), or different samples with different polarities are
used (gradient technique).
- The separated molecules can further be analyzed for
various purposes.
Uses of Column chromatography
- Column chromatography is routinely used for the
separation of impurities and purification of various biological mixtures.
- This technique can also be used for the isolation
of active molecules and metabolites from various samples.
- Column chromatography is increasingly used for the
detection of drugs in crude extracts.
Examples of Column chromatography
- Extraction of pesticides from solid food samples of
animal origin containing lipids, waxes, and pigments.
- Synthesis of Pramlintide which is an analog of
Amylin, a peptide hormone, for treating type 1
and type 2 Diabetics.
- Purification of bioactive glycolipids, showing
antiviral activity towards HSV-1 (Herpes Virus).
5. Flash chromatography
Flash
chromatography is a separation technique where smaller sizes of gel particles
are used as stationary phase, and pressurized gas is used to drive the solvent
through the column.
Principle of Flash chromatography
- The principle of flash chromatography is similar to
that of column chromatography, where the components are separated on the
basis of their differential adsorption to the stationary phase.
- The sample applied is passed by using a pressurized
gas that makes the process faster and more efficient.
- Molecules bind to the stationary phase on the basis
of their affinity while the rest of the solvent is eluted out by applying
the pressured gas which quickens the process.
- Here, the stationary phase is solid, the mobile
phase and the elution solution are liquid, and an additional pressurized
gas is used.
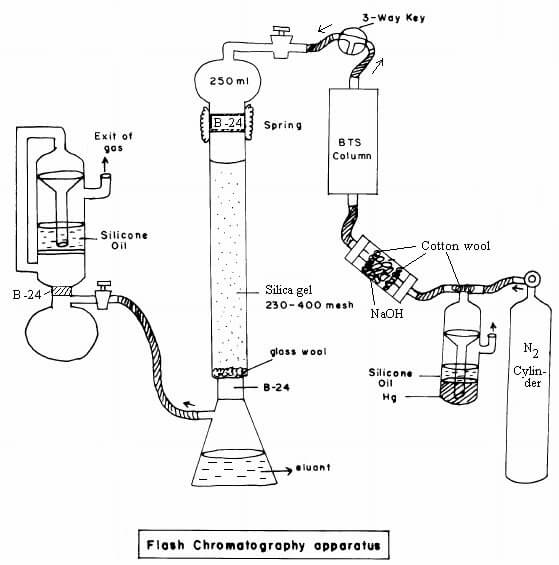
Steps of
Flash chromatography
- The column is prepared by taking a glass tube that
is dried and coated with a thin, uniform layer of stationary phase
(cellulose, silica). The bottom and top of the column are packed with
cotton wool to prevent the gel from escaping.
- Then the sample is prepared by adding the mixture
to the mobile phase. The sample is introduced into the column from the
top, and a pumped sample is used to pass the sample at a constant rate.
- The molecules bound to the column are separated by
elution solution where either solution of the same polarity is used
(isocratic technique), or different samples with different polarities are
used (gradient technique).
- The elution solvent is applied with a constant
minimum pressure required to move the solute down the column.
- The separated molecules can further be analyzed for
various purposes.
Uses of Flash chromatography
- Flash chromatography is used as a rapid and more
efficient method of separation of components of different mixtures.
- It is used for the removal of impurities from crude
extracts of natural and synthetic mixtures.
6. Gas chromatography
Gas
chromatography is a separation technique in which the molecules are separated
on the basis of their retention time depending on the affinity of the molecules
to the stationary phase.
The sample is
either liquid or gas that is vaporized in the injection point.
Principle of Gas chromatography
- Gas chromatography is based on the principle that
components having a higher affinity to the stationary phase have a higher
retention time as they take a longer time to come out of the column.
- However, the components having a higher affinity to
the stationary phase have less retention time as they move along with the
mobile phase.
- The mobile phase is a gas, mostly helium, that
carries the sample through the column.
- The sample once injected in converted into the
vapor stage is then passed through a detector to determine the retention
time.
- The components are collected separately as they
come out of the stationary phase at different times.
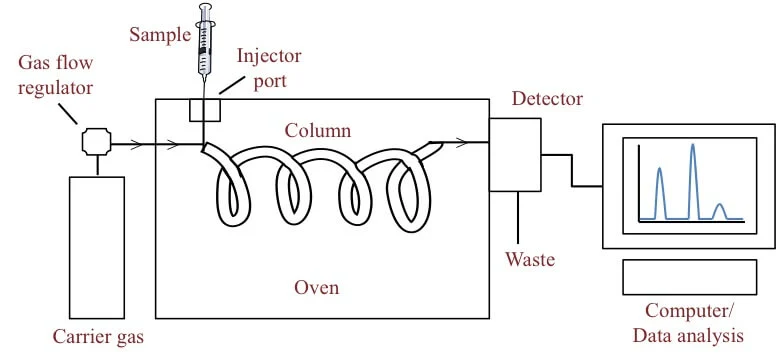
Steps of
Gas chromatography
- The sample is injected into the column where it is
vaporized into a gaseous state. The vapourised component than mixes with
the mobile phase to be carried through the rest of the column.
- The column is set with the stationary phase where
the molecules are separated on the basis of their affinity to the
stationary phase.
- The components of the mixture reach the detector at
different times due to differences in the time they are retained in the
column.
Uses of Gas chromatography
- This technique is used to calculate the
concentration of different chemicals in various samples.
- This is used in the analysis of air pollutants, oil
spills, and other samples.
- Gas chromatography can also be used in forensic
science to identify and quantify various biological samples found in the
crime scene.
Examples of Gas chromatography
- The identification of performance-inducing drug in
the athlete’s urine.
- The separation and quantification of a solid drug
in soil and water samples.
7. Gel filtration chromatography
Gel permeation chromatography/
Size exclusion chromatography/
Molecular sieve chromatography
Gel-filtration
chromatography is a form of partition chromatography used to separate molecules
of different molecular sizes.
This
technique has also frequently been referred to by various other names, including
gel-permeation, gel-exclusion, size- exclusion, and molecular- sieve
chromatography.
Gel filtration chromatography Principle
- Molecules are partitioned between a mobile phase
and a stationary phase as a function of their relative sizes.
- The stationary phase is a matrix of porous polymer
which have pores of specific sizes.
- When the sample is injected with the mobile phase,
the mobile phase occupies the pores of the stationary phase.
- If the size of the molecules is appropriate enough
to enter the pores, they remain in the pores partly or wholly.
- However, molecules with a larger size are retained
from entering the pores, causing them to be moved with the mobile phase,
out of the column.
- If the mobile phase used in an aqueous solution,
the process is termed gel filtration chromatography.
- If the mobile phase used is an organic solvent, it
is termed as gel permeation chromatography.

Gel filtration chromatography steps
- The column is filled with semi-permeable, porous
polymer gel beads with a well-defined range of pore sizes.
- The sample, mixed with the mobile phase, is then
injected into the column from the top of the column.
- The molecules bound to the column are separated by
elution solution where either solution of the same polarity is used
(isocratic technique), or different samples with different polarities are
used (gradient technique).
- Elution conditions (pH, essential ions, cofactors,
protease inhibitors, etc.) can be selected, which will complement the
requirements of the molecule of interest.
Gel filtration chromatography Uses
- One of the principal advantages of gel-filtration
chromatography is that separation can be performed under conditions
specifically designed to maintain the stability and activity of the molecule
of interest without compromising resolution.
- The absence of a molecule-matrix binding step also
prevents unnecessary damage to fragile molecules, ensuring that
gel-filtration separations generally give high recoveries of activity.
- Because of its unique mode of separation,
gel-filtration chromatography has been used successfully in the
purification of proteins and peptides from various sources.
- Gel-filtration chromatography has been used to
separate various nucleic acid species such as DNA, RNA, and tRNA as well
as their constituent bases, adenine, guanine, thymine, cytosine, and
uracil.
Gel filtration chromatography Examples
- The separation of recombinant human granulocyte
colony-stimulating factor (rhG-CSF) from inclusion bodies in high yield by
urea-gradient size-exclusion chromatography.
- The separation of hen egg lysozyme using both
acrylamide- and dextran-based gel columns.
8. High-performance liquid chromatography (HPLC)
High-performance
liquid chromatography is a modified form of column chromatography where the
components of a mixture are separated on the basis of their affinity with the
stationary phase.
Principle of HPLC
- This technique is based on the principle of differential
adsorption where different molecules in a mixture have a varying degree of
interactions with the absorbent present on the stationary phase.
- The molecules having higher affinity remain
adsorbed for a longer time decreasing their speed of movement through the
column.
- However, the molecules with lower affinity move
with a faster movement, thus allowing the molecules to be separated in
different fractions.
- This process is slightly different from the column
chromatography as in this case; the solvent is forced under high pressures
of up to 400 atmospheres instead of allowing it to drip down under
gravity.
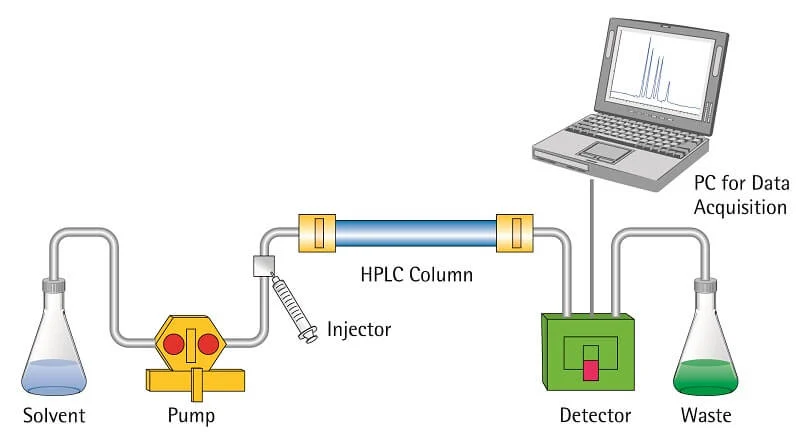
Steps of
HPLC
- The column is prepared by taking a glass tube that
is dried and coated with a thin, uniform layer of stationary phase
(cellulose, silica).
- Then the sample is prepared by adding the mixture
to the mobile phase. The sample is introduced into the column from the
top, and a high-pressure pump is used to pass the sample at a constant
rate.
- The mobile phase then moves down to a detector that
detects molecules at a certain absorbance wavelength.
- The separated molecules can further be analyzed for
various purposes.
Uses of HPLC
- High-performance liquid chromatography is used in
the analysis of pollutants present in environmental samples.
- It is performed to maintain product purity and
quality control of various industrial productions.
- This technique can also be used to separate
different biological molecules like proteins and nucleic acids.
- The increased speed of this technique makes the
process faster and more effective.
Example of HPLC
- High-performance liquid chromatography has been
performed to test the efficiency of different antibodies against diseases
like Ebola.
9. Hydrophobic interaction chromatography
Hydrophobic
interaction chromatography is the separation technique that separates molecules
on the basis of their degree of hydrophobicity.
Principle of Hydrophobic interaction chromatography
- The principle of hydrophobic interaction
chromatography is based on the interaction between two molecules with
hydrophobic groups.
- Here, the stationary phase is solid support applied
with both hydrophobic and hydrophilic groups.
- The solvent molecules containing hydrophobic
regions interact with the hydrophobic groups, thus separating them from
the molecules with hydrophilic groups.
- The interaction is then reversed by applying an
elution solution with decreasing salt gradient, which causes the molecules
with hydrophobic groups to be separated from the stationary phase.

Steps of
Hydrophobic interaction chromatography
- The column is prepared with a glass tube applied
with solid support like silica gel, upon which hydrophobic groups like
phenyl, octyl butyl, are attached.
- The sample is prepared by adding the mixture to the
mobile phase.
- The sample is then injected into the column from
the top of the column.
- The molecules with hydrophobic groups form an
interaction with the hydrophobic groups of the stationary phase. In
contrast, the molecules without such groups move out of the column with
the mobile phase.
- Then a particular elution solution with decreasing
salt gradient is then passed into the column that removes the bound
molecules from the stationary phase.
Uses of Hydrophobic interaction chromatography
- Hydrophobic interaction chromatography is extremely
important for the separation of proteins with hydrophobic groups.
- This technique is more appropriate than other
methods, as this technique results in minimum denaturation activities.
- Similarly, this method can also be applied to the
separation of other organic compounds with hydrophobic groups.
- This allows the separation of hydrophilic and
hydrophobic biological molecules from each other.
Example of Hydrophobic interaction chromatography
- The separation of plant proteins from the crude
extracts.
10. Ion exchange chromatography
Ion exchange
chromatography is the separation technique for charged molecules by their
interaction with the oppositely charged stationary phase in the form of
ion-exchange resin.
Principle of Ion exchange chromatography
- This technique is based on the principle of
attraction of charged resin and the oppositely charged analyte. Here the
exchange of negatively/ positively charged ions takes place to remove the
charged molecules.
- The stationary phase is first coated with
particular charges where the components of the mixture with opposite
charges will bind.
- A cation or anion exchange resin with a higher
affinity to the charged components then binds the components, displacing
the oppositely charged resin.
- The cation or anion exchange resin-component
complex then is removed by using different buffers.

Steps of
Ion exchange chromatography
- A column packed with charged resin that can either
be positively charged or negatively charged is taken as the stationary
phase.
- The mixture with the charged particles is then
passed down the column where the charged molecules bind to the oppositely
charged resins.
- If a cation exchange resin is used, the positively
charged molecules now bind to the cation exchange resin displacing the
negatively charged resin.
- Similarly, if an anion exchange resin is used, the
negatively charged molecules bind to the anion exchange resin displacing
the positively charged resin.
- Now an appropriate buffer is applied to the column
to separate the complex of charged exchange resins and the charged
molecules.
Uses of Ion exchange chromatography
- Ion exchange chromatography is used in the
purification of water where the positively charged ions are replaced by
hydrogen ions, and the negatively charged ions are replaced by hydroxyl
ions.
- This method also works as an effective method for
the analysis of the products formed after hydrolysis of nucleic acids.
- The separation of metals and other inorganic
compounds is also facilitated by the ion-exchange chromatography.
Examples of Ion exchange chromatography
- The separation of positively charged lanthanoid
ions obtained from the earth’s crust.
- The separation of proteins from the crude mixture
obtained from the blood serum.
11. Liquid chromatography
Liquid
chromatography is a separation technique where the mobile phase used is liquid,
and the separation can take place either in a column or a plain surface.
Principle of Liquid chromatography
- The process of liquid chromatography is based on
the principle for the affinity of the molecules to the mobile phase.
- If the components to be separated have a higher
affinity to the mobile phase, the molecules move along with the mobile
phase and come out of the column faster.
- However, if the components have a lower degree of
interaction with the mobile phase, the molecules move slowly and thus come
out of the column later.
- Thus, if two molecules in a mixture have different
polarities and the mobile phase is of a distinct polarity, the two
molecules will move at different speeds through the stationary phase.

Steps of
Liquid chromatography
- The column or paper is prepared where the
stationary phase (cellulose or silica) is applied on the solid support.
- The sample is added to the liquid mobile phase,
which is then injected into the chromatographic system.
- The mobile phase moves through the stationary phase
before coming out of the column or the edge of the paper.
- An elution solution is applied to the system to
separate the molecules from the stationary phase.
Uses of Liquid chromatography
- Liquid chromatography is an effective method for
the separation of a colored solution as they form two separate bands after
separation.
- This method can also be used over other techniques
as it is quite simple and less expensive.
- It can be used for the separation of solid
molecules that are insoluble in water.
Examples of Liquid chromatography
- High-performance liquid chromatography is a
modified form of liquid chromatography that is used in the research
regarding biological molecules.
12. Paper chromatography
Paper
chromatography is a separation technique where the separation is performed on a
specialized paper.
Principle of Paper chromatography
- Paper chromatography is of two types based on two
different principles.
- The first is the paper adsorption chromatography
that is based on the varying degree of interaction between the molecules
and the stationary phase.
- The molecules having higher affinity remain
adsorbed for a longer time decreasing their speed of movement through the
column.
- However, the molecules with lower affinity move
with a faster movement, thus allowing the molecules to be separated in
different fractions.
- The second type of paper chromatography is the paper
partition chromatography. It is based on the principle that the moisture
on the cellulose paper acts as a stationary phase for the molecules moving
with the mobile phase.
- The separation of the molecules is thus based on
how strongly they adsorb onto the stationary phase.
- An additional concept of ‘retention factor’ is
applied during the separation of molecules in the paper chromatography.
- The retention value for a molecule is determined as
a ratio of distance traveled by the molecule to the distance traveled by
the mobile phase.
- The retention value of different molecules can be
used to differentiate those molecules.

Steps of
Paper chromatography
- The stationary phase is selected as a fine quality
cellulosic paper.
- Different combinations of organic and inorganic
solvents are taken as the mobile phase.
- About 2-200 µl of the sample solution is injected
at the baseline of the paper, and it is allowed to air dry.
- The sample loaded paper is then carefully dipped
into the mobile phase not more than the height of 1 cm.
- After the mobile phase reaches near the edge of the
paper, the paper is taken out.
- The retention factor is calculated, and the
separated components are detected by different techniques.
Uses of Paper chromatography
- Paper chromatography is performed to detect the
purity of various pharmaceutical products.
- It can also be employed to detect contamination in
various samples, like food and beverages.
- This method can also be used for the separation of
impurities from various industrial products.
- The analysis of the reaction mixtures in chemical
labs is also conducted via paper chromatography.
Examples of Paper chromatography
- Paper chromatography is used in the separation of
mixtures of inks or other colored drinks.
13. Reverse-phase chromatography
Reverse-phase
chromatography is a liquid chromatography technique where the separation of
molecules is achieved through hydrophobic interaction between the liquid mobile
phase and the stationary phase.
Principle of Reverse-phase chromatography
- The principle of reverse phase chromatography is
based on the interaction between two molecules with hydrophobic groups.
- Here, the stationary phase is solid support applied
with both hydrophobic and hydrophilic groups.
- The solvent molecules containing hydrophobic regions
interact with the hydrophobic groups, thus separating them from the
molecules with hydrophilic groups.
- The interaction is then reversed by applying an
elution solution with decreasing salt gradient, which causes the molecules
with hydrophobic groups to be separated from the stationary phase.
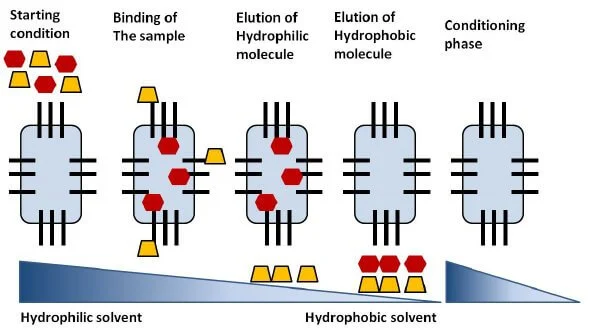
Steps of
Reverse-phase chromatography
- The column is prepared with a glass tube applied
with solid support like silica gel, upon which hydrophobic groups like
phenyl, octyl butyl, are attached.
- The sample is prepared by adding the mixture to the
mobile phase of organic and inorganic solvents.
- The sample is then injected into the column from
the top of the column.
- The molecules with hydrophobic groups form an
interaction with the hydrophobic groups of the stationary phase. In
contrast, the molecules without such groups move out of the column with
the mobile phase.
- Then a particular elution solution with decreasing
salt gradient is then passed into the column that removes the bound
molecules from the stationary phase.
Uses of Reverse-phase chromatography
- Reverse chromatography, in combination with
high-performance liquid chromatography, is increasingly used for the
separation of biomolecules.
- This is also used in the study of the analysis of
drugs, metabolites, and active molecules.
- It can also be used to remove impurities from
various environmental samples.
Examples of Reverse-phase chromatography
- Hydrophobic interaction chromatography is an
example of reverse phase chromatography where this technique is used to
separate proteins from their mixtures.
14. Thin-layer chromatography (TLC)
Thin-layer
chromatography is a separation technique where the stationary phase is applied
as a thin layer on a solid support plate with a liquid mobile phase.
Principle of Thin-layer chromatography (TLC)
- This chromatography technique is based on the
principle that components of a mixture are separated when the component
having an affinity towards the stationary phase binds to the stationary
phase. In contrast, other components are eluted with the mobile phase.
- The substrate/ ligand is bound to the stationary
phase so that the reactive sites for the binding of components are
exposed.
- Now, the mixture is passed through the mobile phase
where the components with binding sites for the substrate bind to the
substrate on the stationary phase while the rest of the components are
eluted out with the mobile phase.
- After separation, the molecules are seen as spots
at a different location throughout the stationary phase.
- The detection of molecules is performed by various
techniques.
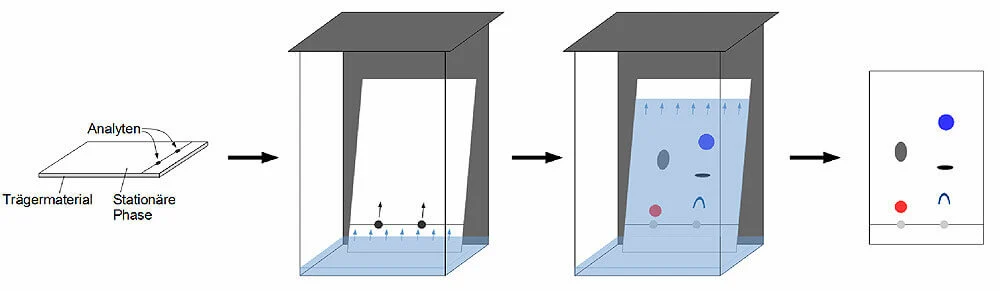
Steps of
Thin-layer chromatography (TLC)
- The stationary phase is uniformly applied on the
solid support (glass, thin plate or aluminum foil) and dried.
- The sample is injected as spots on the stationary
phase about 1 cm above the edge of the plate.
- The sample loaded plate is then carefully dipped
into the mobile phase not more than the height of 1 cm.
- After the mobile phase reaches near the edge of the
plate, the plate is taken out.
- The retention factor is calculated as in paper
chromatography, and the separated components are detected by different
techniques.
Uses of Thin-layer chromatography (TLC)
- Thin-layer chromatography is routinely performed in
laboratories to identify different substances present in a mixture.
- This technique helps in the analysis of fibers in
forensics.
- TLC also allows the assay of various pharmaceutical
products.
- It aids in the identification of medicinal plants and their composition.








