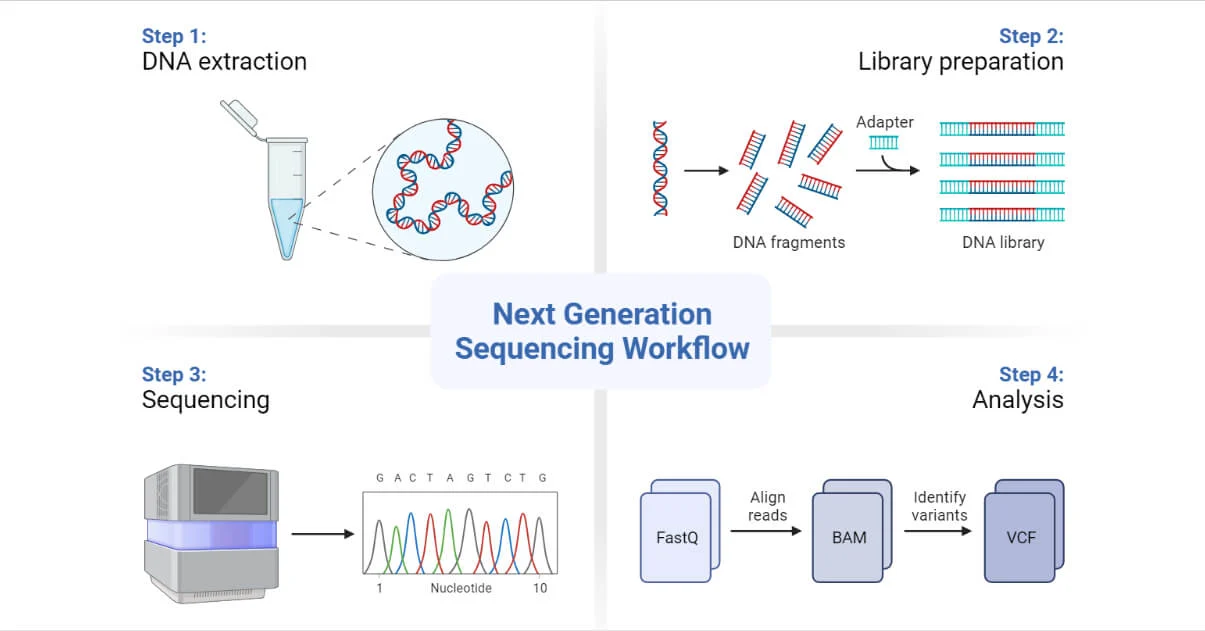
Next Generation Sequencing (NGS) is a robust platform that has enabled the sequencing of thousands to millions of DNA molecules simultaneously.
Next-generation sequencing (NGS), also known as
high-throughput sequencing, is the catch-all term used to describe a number of
different modern sequencing technologies.
The high
demand for low-cost sequencing has driven the development of high-throughput
sequencing, which produces thousands or millions of sequences at
once.
They are
intended to lower the cost of DNA sequencing beyond what is possible with
standard dye-terminator methods.
Thus, these
recent technologies allow us to sequence DNA and RNA much more quickly and
cheaply than the previously used Sanger sequencing and as such, have
revolutionized the study of genomics and molecular biology.
Classified to different generations, NGS has led to overcoming the limitations of conventional DNA sequencing methods and has found usage in a wide range of molecular biology applications.

The
generations it is classified into include:
First Generation
- Sanger Sequencing
Second Generation Sequencing
- Pyrosequencing
- Sequencing by Reversible Terminator Chemistry
- Sequencing by Ligation
Third Generation Sequencing
- Single Molecule Fluorescent Sequencing
- Single Molecule Real Time Sequencing
- Semiconductor Sequencing
- Nanopore Sequencing
Fourth Generation Sequencing
Aims conducting genomic analysis directly in the cell.

Next-Generation Sequencing Types
Lynx therapeutics’ massively parallel signature sequencing (MPSS)
- It is considered as the first of the
“next-generation” sequencing technologies.
- MPSS was developed in the 1990s at Lynx
Therapeutics, a company founded in 1992 by Sydney Brenner and Sam Eletr.
- MPSS is an ultra high throughput sequencing
technology.
- When applied to expression profile, it reveals
almost every transcript in the sample and provide its accurate expression
level.
- MPSS was a bead-based method that used a complex
approach of adapter ligation followed by adapter decoding, reading the
sequence in increments of four nucleotides; this method made it
susceptible to sequence-specific bias or loss of specific sequences.
- However, the essential properties of the MPSS
output were typical of later “next-gen” data types, including hundreds of
thousands of short DNA sequences.
- In the case of MPSS, these were typically used for sequencing cDNA for measurements of gene expression levels.
Polony sequencing
- It is an
inexpensive but highly accurate multiplex sequencing technique that can be used
to read millions of immobilized DNA sequences in parallel.
- This
technique was first developed by Dr. George Church in Harvard Medical college.
- It combined
an in vitro paired-tag library with emulsion PCR, an automated microscope, and
ligation-based sequencing chemistry to sequence an E. coli genome at an
accuracy of > 99.9999% and a cost approximately 1/10 that of Sanger
sequencing.
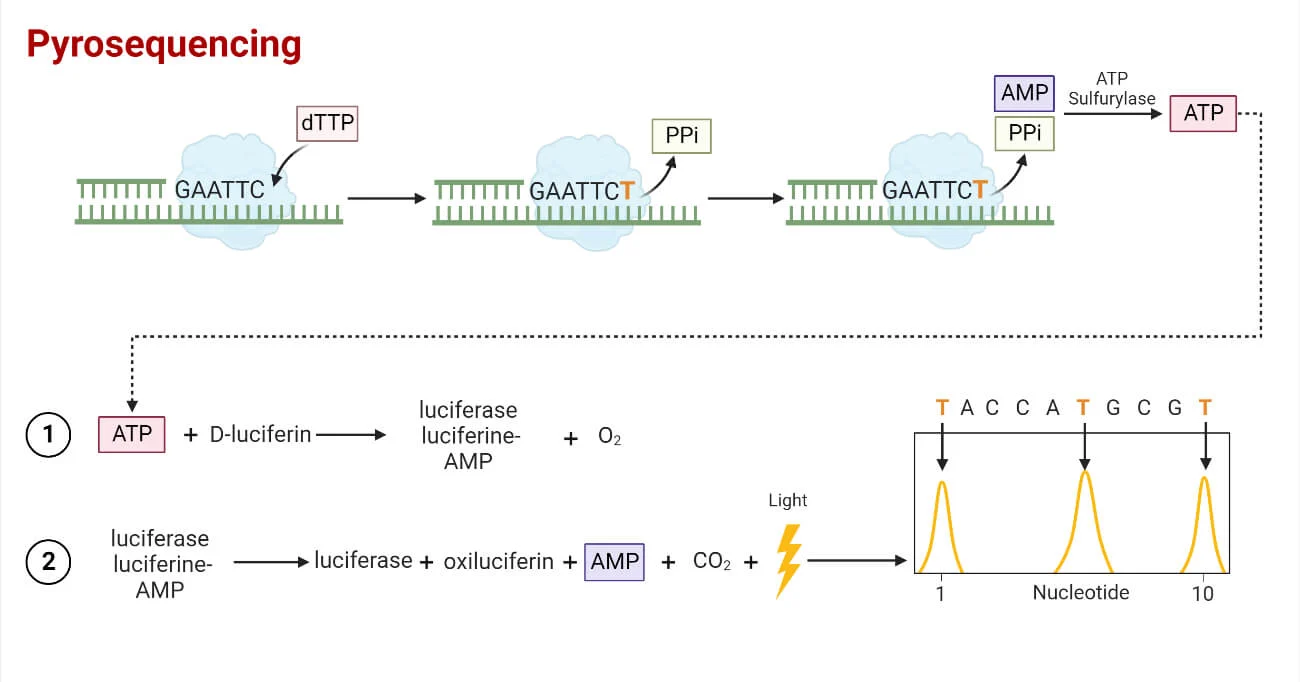
Pyrosequencing
- A parallelized version of pyrosequencing was
developed by 454 Life Sciences, which has since been acquired by Roche
Diagnostics.
- The method amplifies DNA inside water droplets in
an oil solution (emulsion PCR), with each droplet containing a single DNA
template attached to a single primer-coated bead that then forms a clonal
colony.
- The sequencing machine contains many picolitre-volume
wells each containing a single bead and sequencing enzymes.
- Pyrosequencing uses luciferase to generate light
for detection of the individual nucleotides added to the nascent DNA, and
the combined data are used to generate sequence read-outs.
- This technology provides intermediate read length
and price per base compared to Sanger sequencing on one end and Solexa and
SOLiD on the other.
Illumina (Solexa) sequencing
- Solexa developed a sequencing technology based on
dye terminators.
- In this method, DNA molecule are first attached to
primers on a slide and amplified. This is known as bridge amplification.
- Unlike pyrosequencing, the DNA can only be extended
one nucleotide at a time.
- A camera takes images of the fluorescently labeled
nucleotides, then the dye along with the terminal 3′ blocker is chemically
removed from the DNA, allowing the next cycle to commence.
SOLiD sequencing
- The technology for sequencing used in ABISolid
sequencing is oligonucleotide ligation and detection.
- In this, a pool of all possible oligonucleotides of
fixed length are labelled according to the sequenced position.
- This sequencing results to the sequences of
quantities and lengths comparable to illumine sequencing.
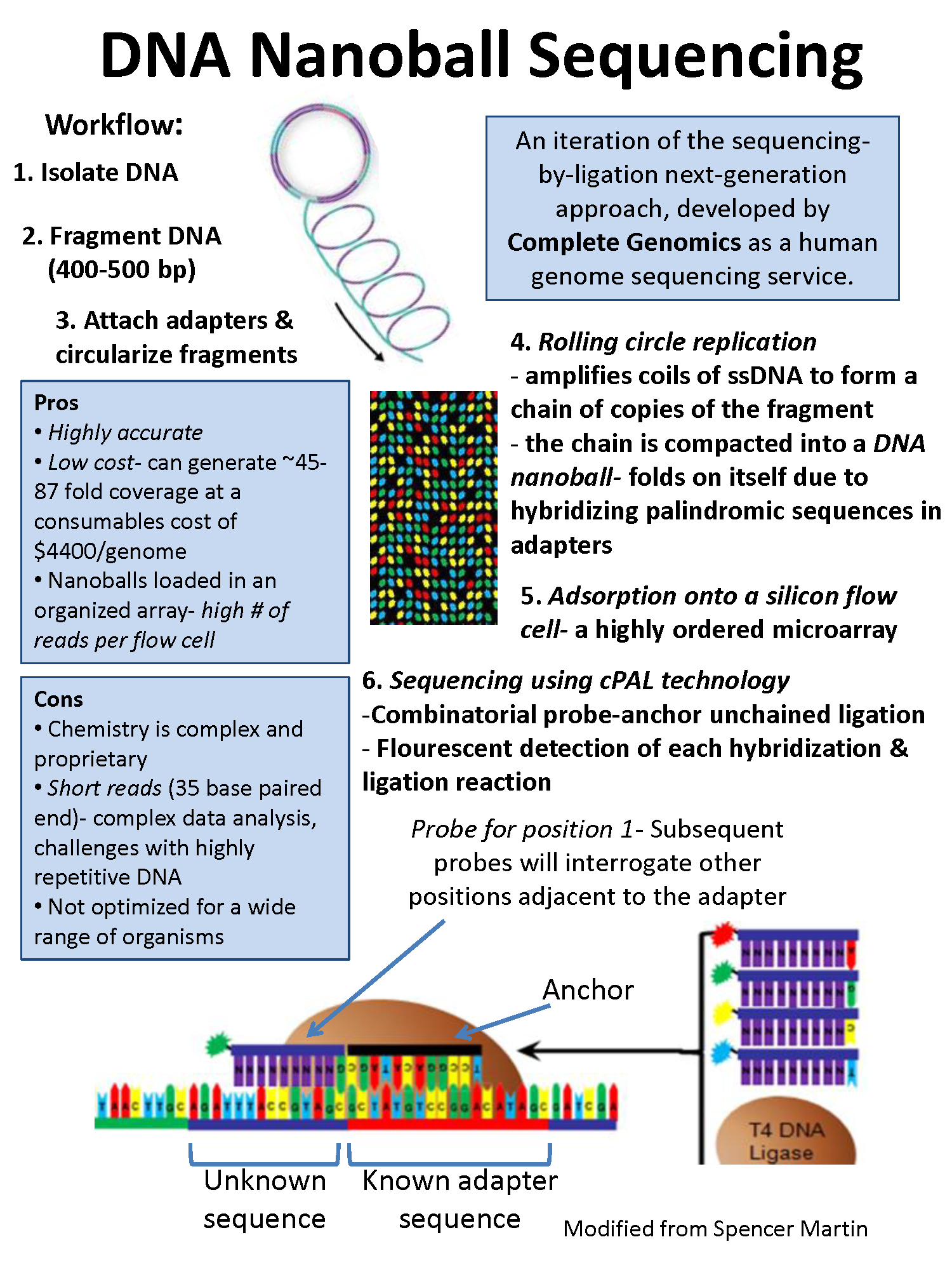
DNA nanoball sequencing
- It is high throughput sequencing technology that is
used to determine the entire genomic sequence of an organism.
- The method uses rolling circle replication to
amplify fragments of genomic DNA molecules.
- This DNA sequencing allows large number of DNA
nanoballs to be sequenced per run and at low reagent cost compared to
other next generation sequencing platforms.
- However, only short sequences of DNA are determined
from each DNA nanoball which makes mapping the short reads to a reference
genome difficult.
- This technology has been used for multiple genome
sequencing projects and is scheduled to be used for more.
Helioscope single molecule sequencing
- Helioscope sequencing uses DNA fragments with added
polyA tail adapters, which are attached to the flow cell surface.
- The next steps involve extension-based sequencing
with cyclic washes of the flow cell with fluorescently labeled
nucleotides.
- The reads are performed by the Helioscope
sequencer.
- The reads are short, up to 55 bases per run, but
recent improvement of the methodology allows more accurate reads of
homopolymers and RNA sequencing.
Single molecule SMRT sequencing
- SMRT sequencing is based on the sequencing by
synthesis approach.
- The DNA is synthesisd in so called zero-mode
wave-guides (ZMWs) – small well-like containers with the capturing tools
located at the bottom of the well.
- The sequencing is performed with use of unmodified
polymerase and fluorescently labelled nucleotides flowing freely in the
solution.
- The wells are constructed in a way that only the
fluorescence occurring by the bottom of the well is detected.
- The fluorescent label is detached from the
nucleotide at its incorporation into the DNA strand, leaving an unmodified
DNA strand.
- The SMTR technology allows detection of nucleotide
modifications. This happens through the observation of polymerase
kinetics.
- This approach allows reads of 1000 nucleotides.
Single molecule real time (RNAP) sequencing

- This method is based on RNA polymerase (RNAP),
which is attached to a polystyrene bead, with distal end of sequenced DNA
is attached to another bead, with both beads being placed in optical
traps.
- RNAP motion during transcription brings the beads
in closer and their relative distance changes, which can then be recorded
at a single nucleotide resolution.
- The sequence is deduced based on the four readouts with lowered concentrations of each of the four nucleotide types.